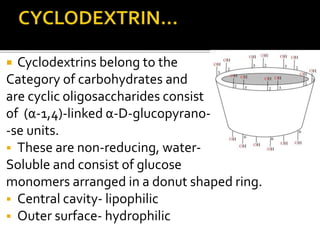
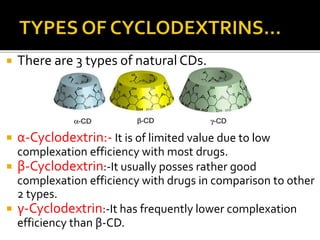
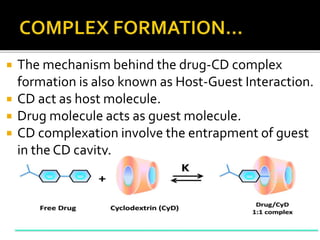
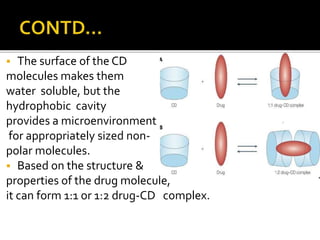
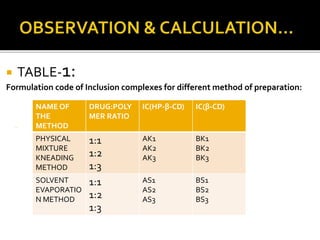
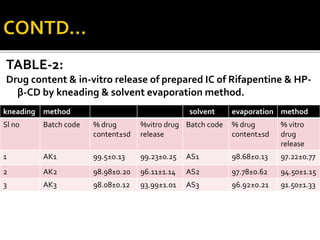
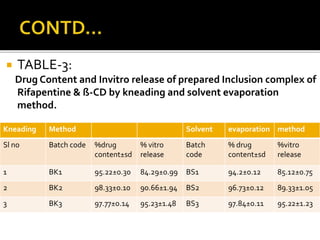
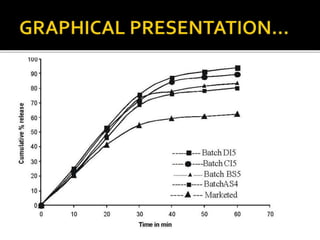
This document summarizes a seminar presentation on solubilization by inclusion complexation. It discusses how inclusion complexation can be used as a solubility enhancement technique. It defines solubility and solubilization. Cyclodextrins are introduced as host molecules that can form inclusion complexes with guest drug molecules to improve their solubility. Different cyclodextrins and mechanisms of complex formation are described. Methods for preparing drug-cyclodextrin complexes like kneading, melting, co-evaporation and freeze drying are summarized. Limitations of complexation are noted. Examples of studies forming inclusion complexes of rifapentine and acyclovir with cyclodextrins are presented, showing improved solubility and dissolution rates