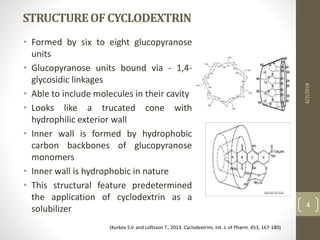
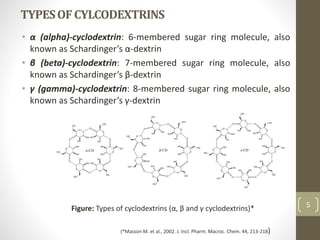
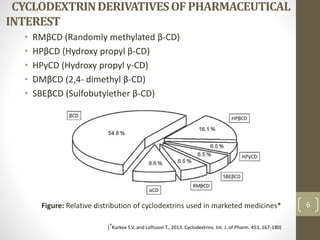
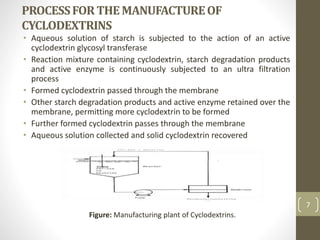
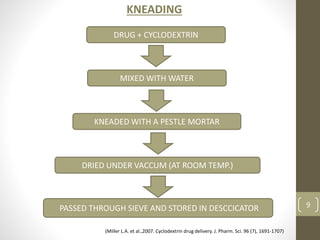
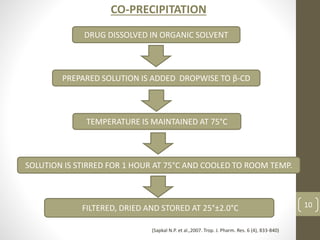
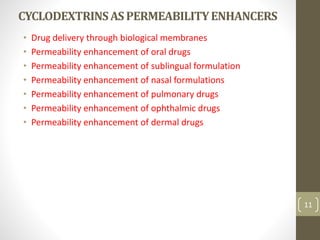
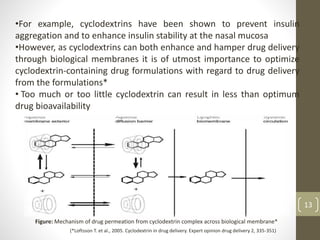
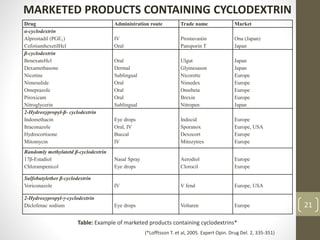
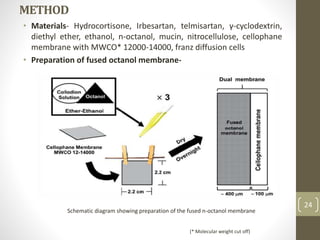
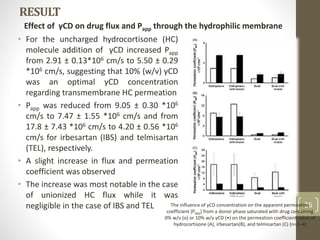
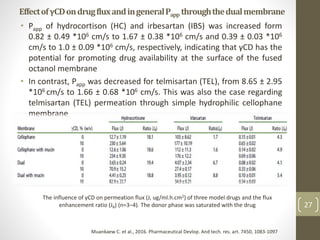
Cyclodextrins are cyclic oligosaccharides that can form inclusion complexes with drug molecules to improve their solubility and permeability. There are three main types of cyclodextrins - alpha, beta, and gamma - as well as various chemically modified derivatives used pharmaceutically. Cyclodextrins act as permeability enhancers for drugs administered orally, sublingually, nasally, pulmonarily, ophthalmically, and dermally by increasing solubility and dissolution rate. Approximately 30 marketed products contain cyclodextrins to improve drug properties. Cyclodextrins are an important tool in pharmaceutical formulations.