The document outlines 7 general rules for drawing Lewis structures:
1. Count the total valence electrons from the group numbers of each atom. Add/subtract electrons for ions.
2. Calculate the total electrons needed for each atom to have an octet or doublet.
3. Subtract the total valence electrons from the total electrons needed to get the bonding electrons.
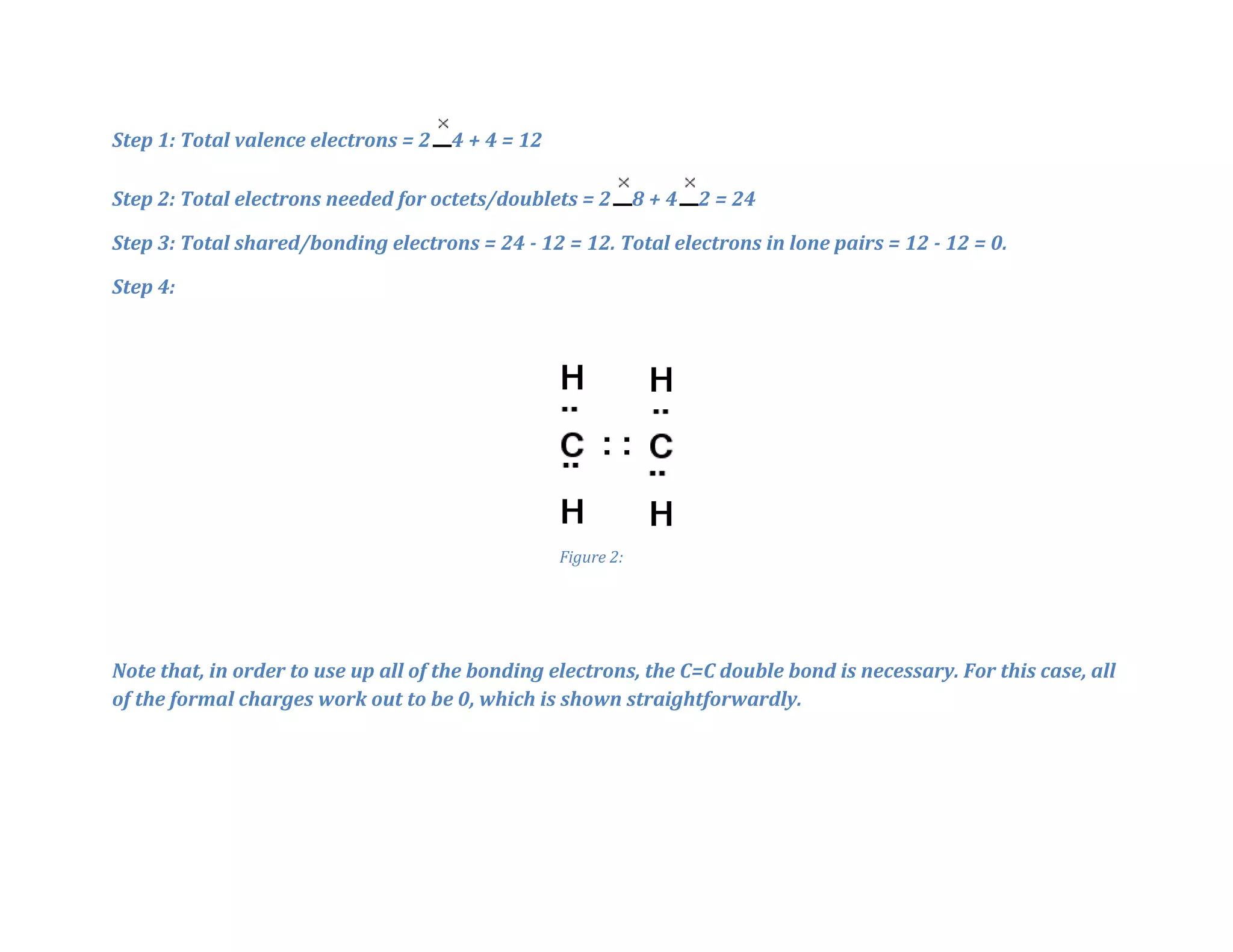
4. Assign two electrons to each bond. Assign remaining electrons to double/triple bonds if possible.
5. Assign any electrons left as lone pairs to complete octets, except for hydrogen.
6. Calculate and note the formal charges to ensure they add up to the total charge on the molecule/ion.