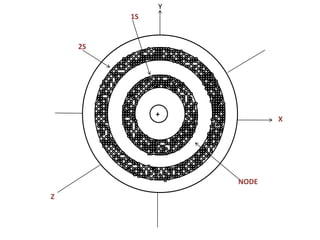
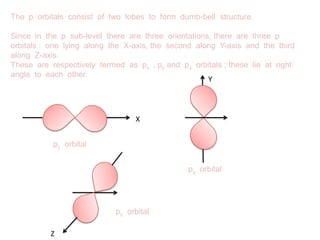
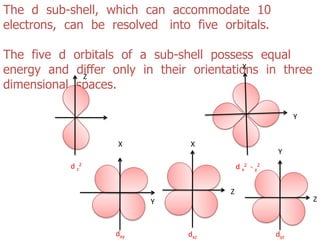
The document discusses the shapes of atomic orbitals. It explains that electron clouds are not uniform but densest where electrons are most likely to be found. S orbitals are spherical around the nucleus, with larger orbitals having higher principal quantum numbers n. P orbitals have a dumbbell shape with three orientations (px, py, pz) at right angles. D orbitals consist of five shapes, two along the axes and three between the axes, with differing probability distributions.