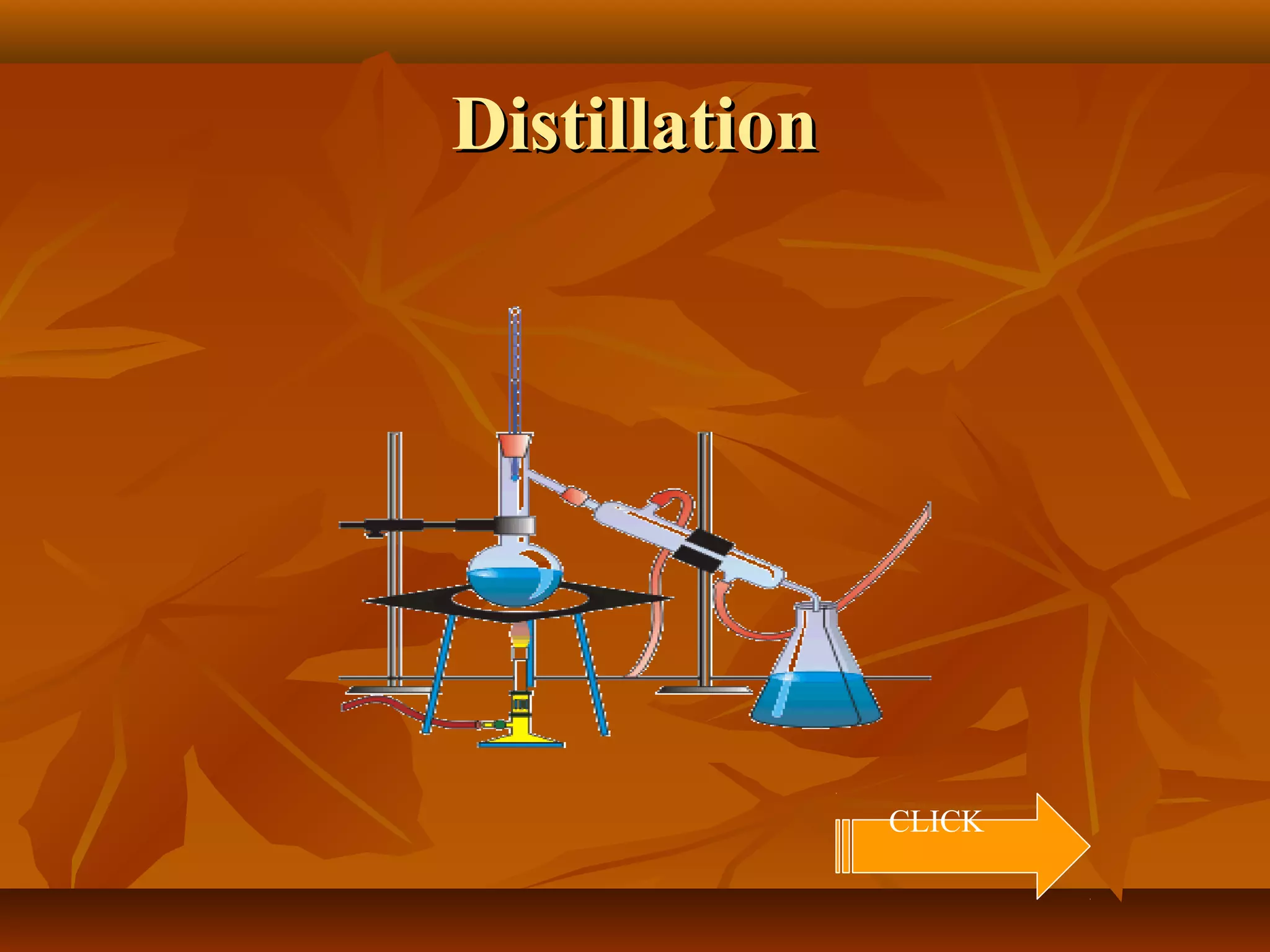
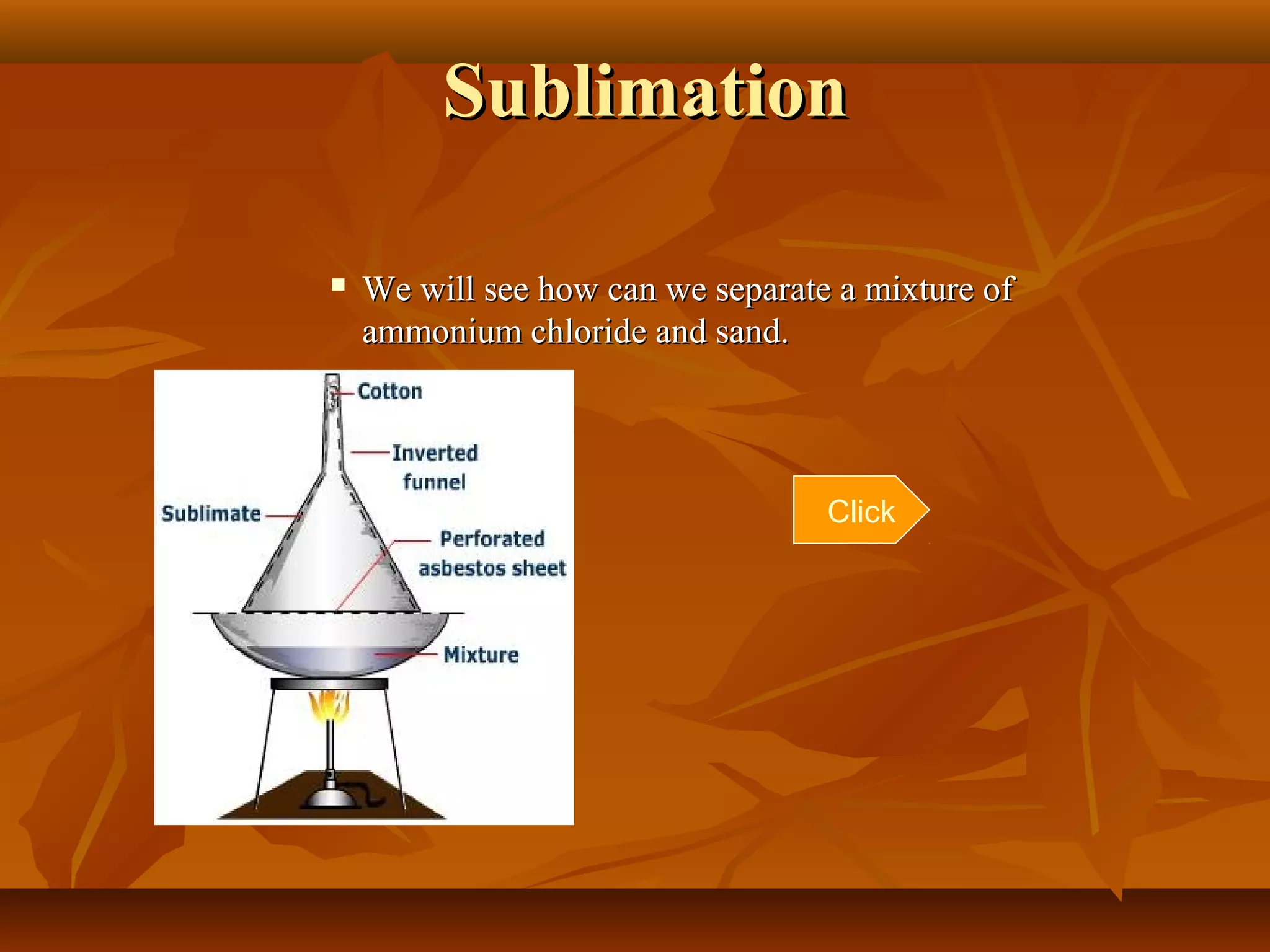
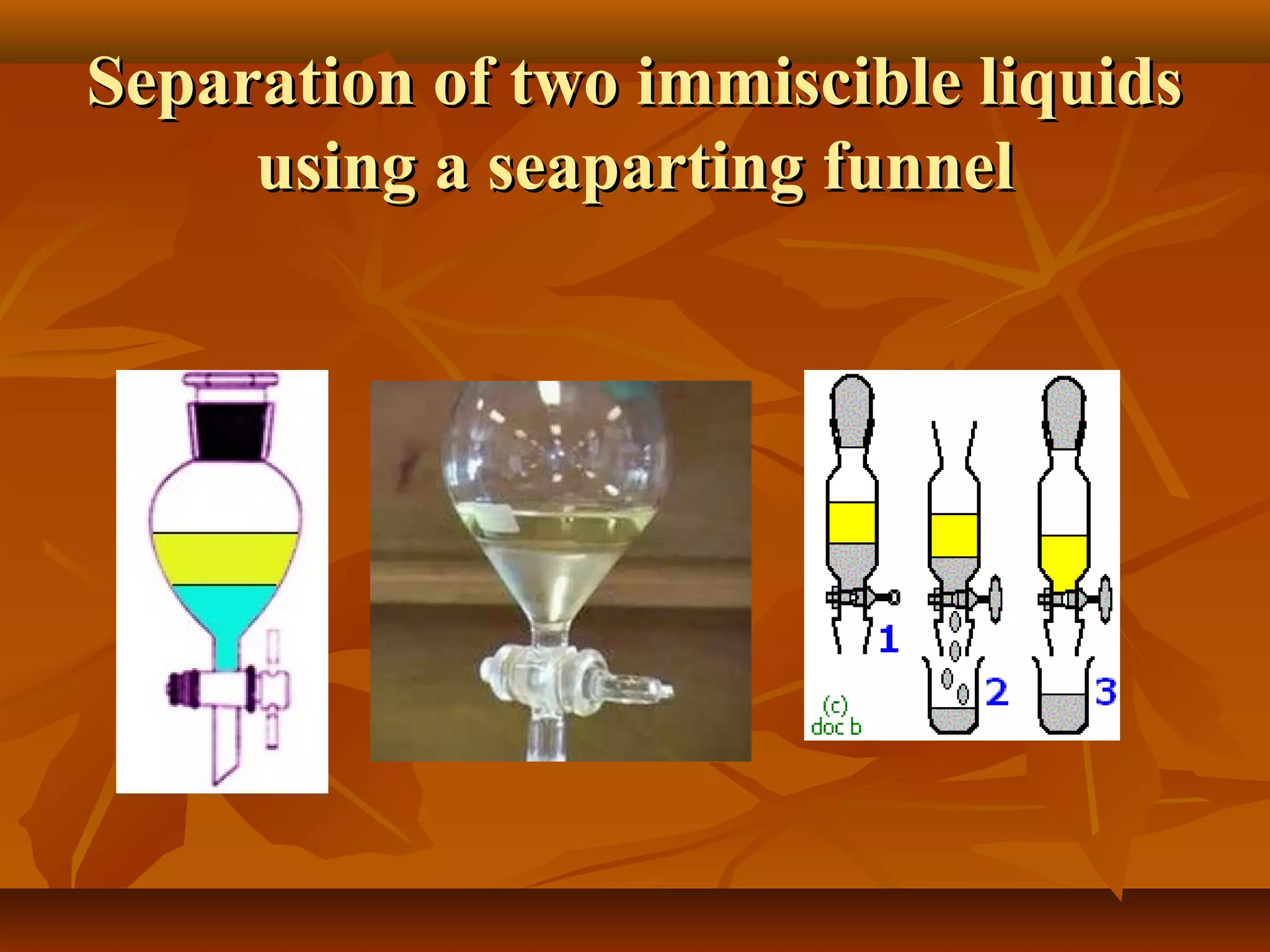
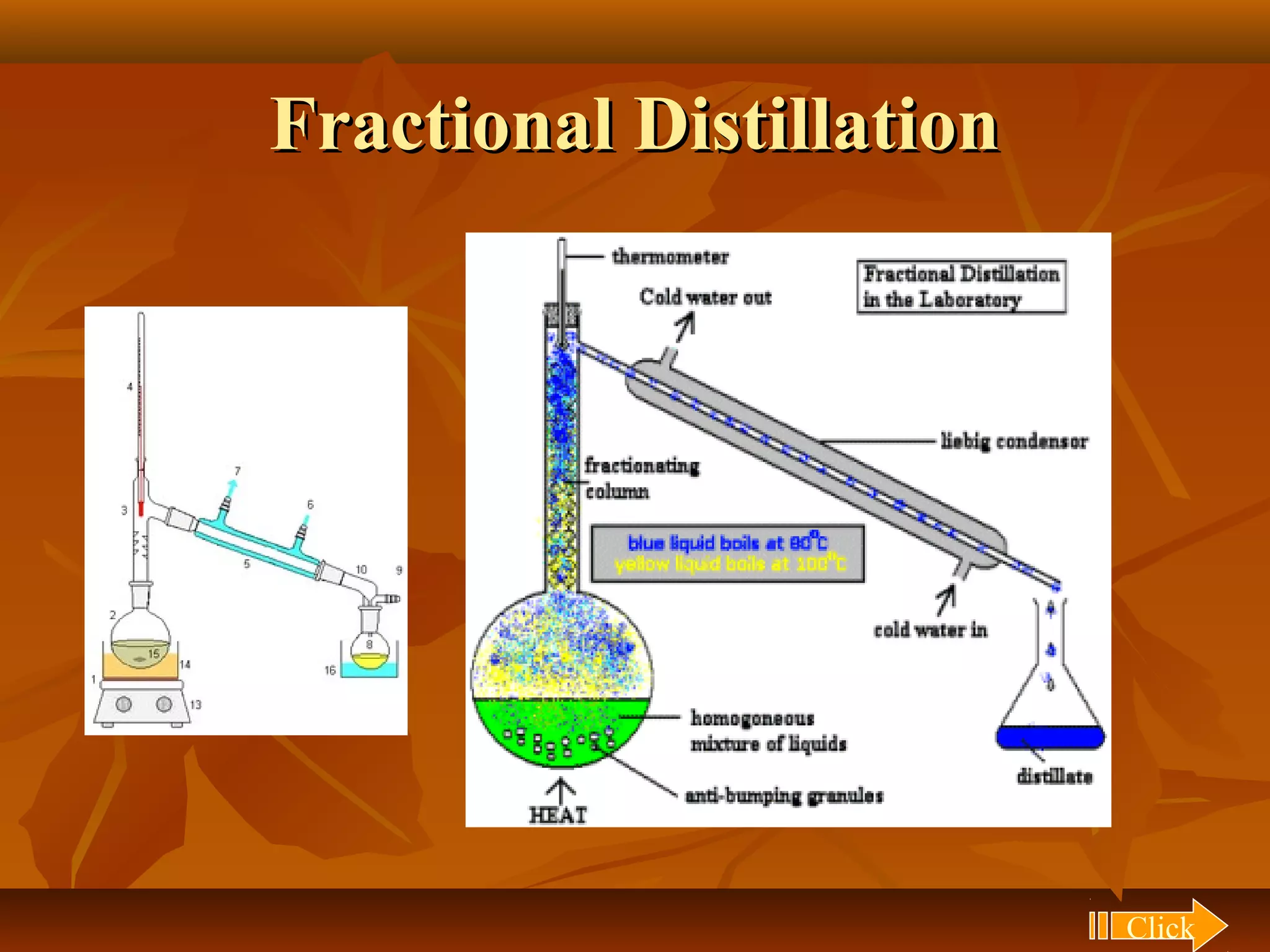
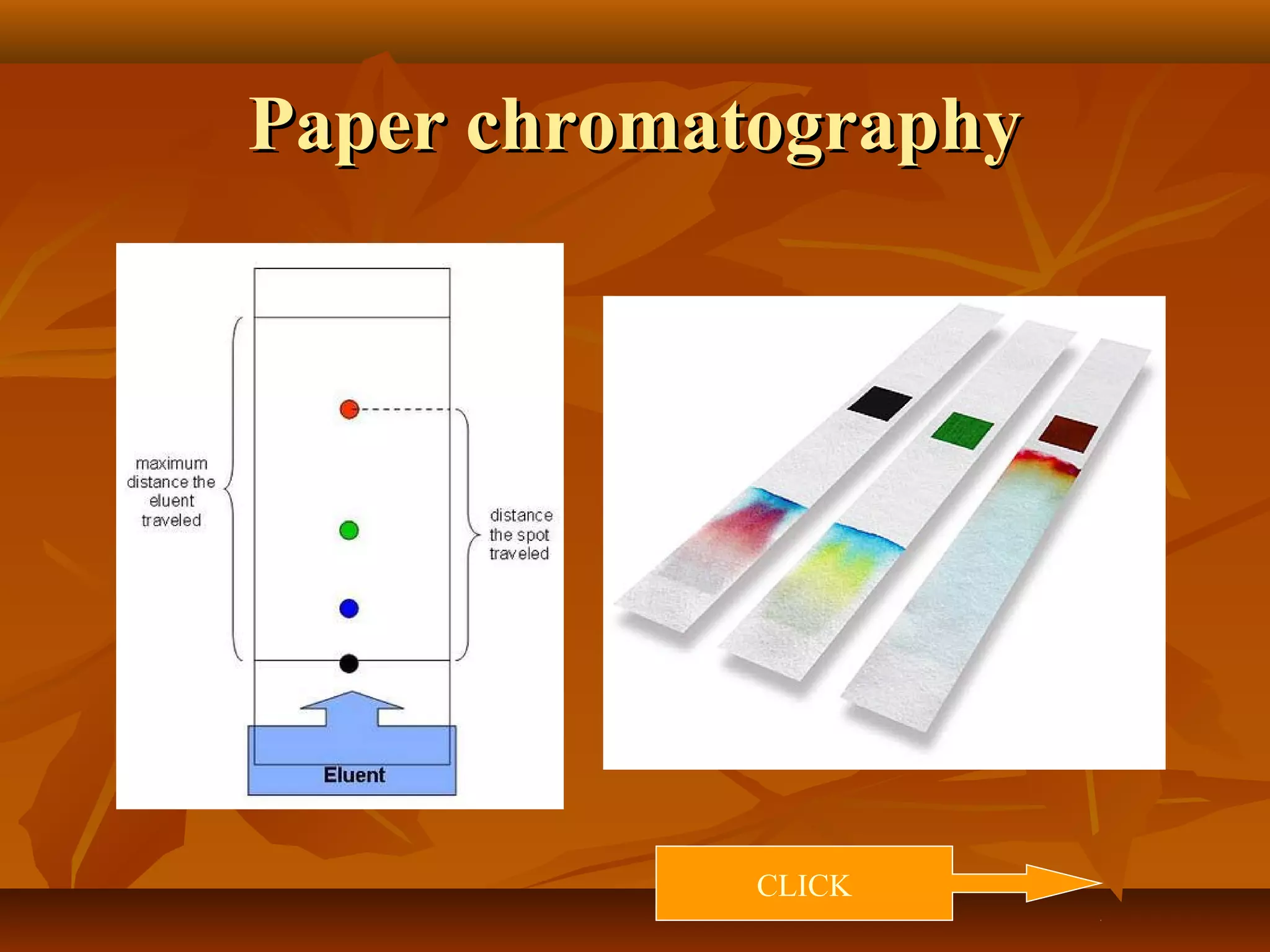
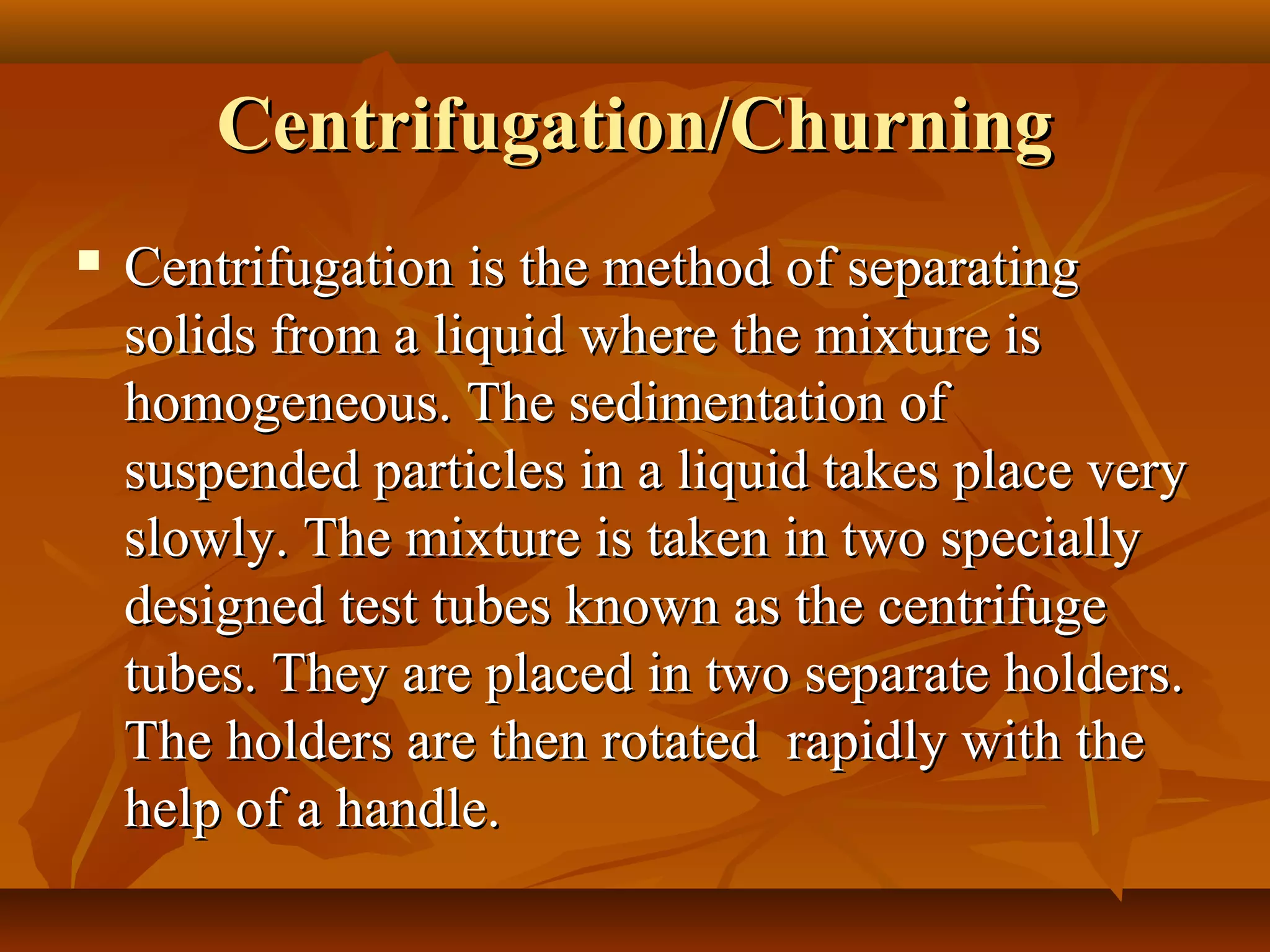
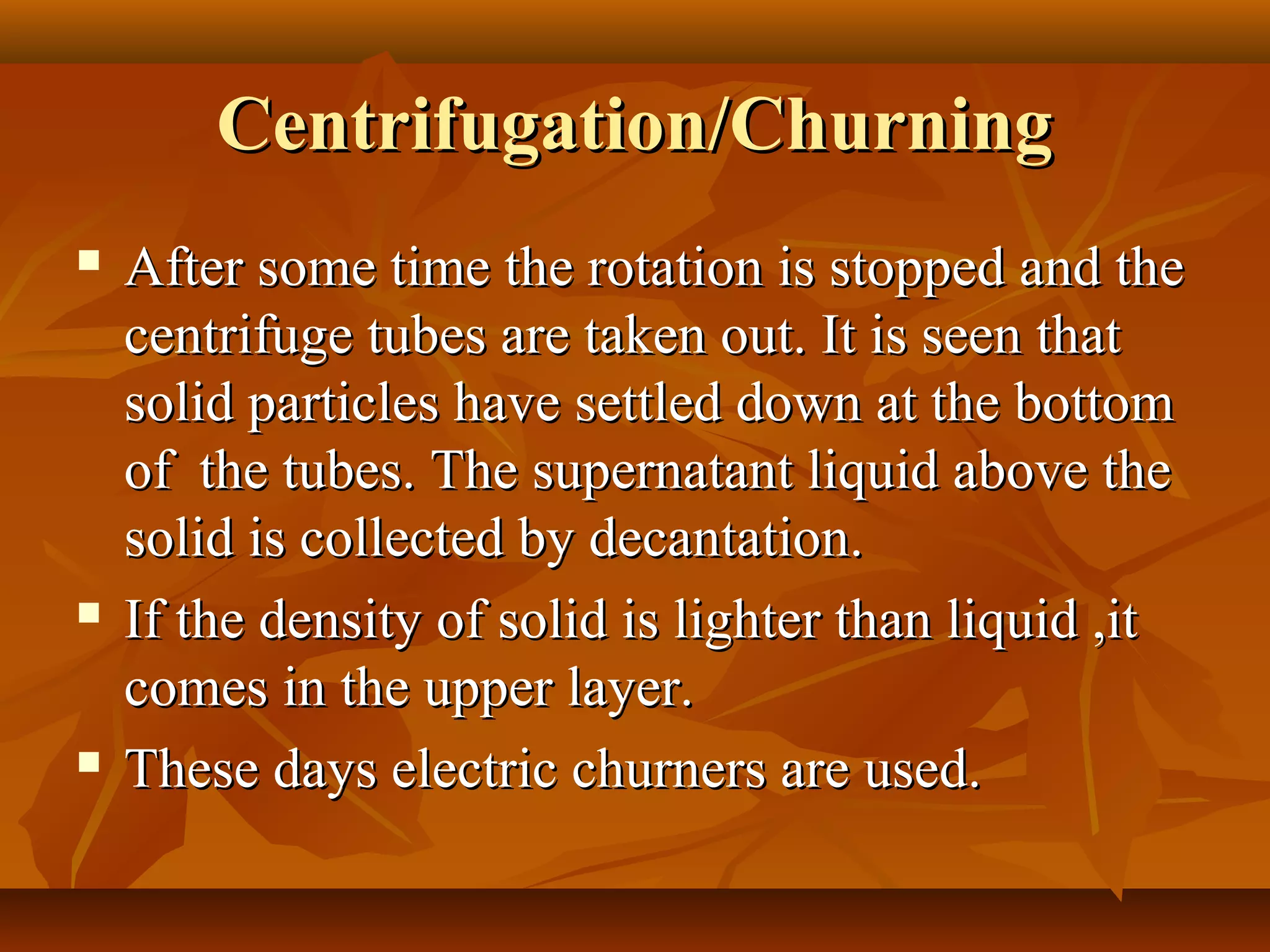
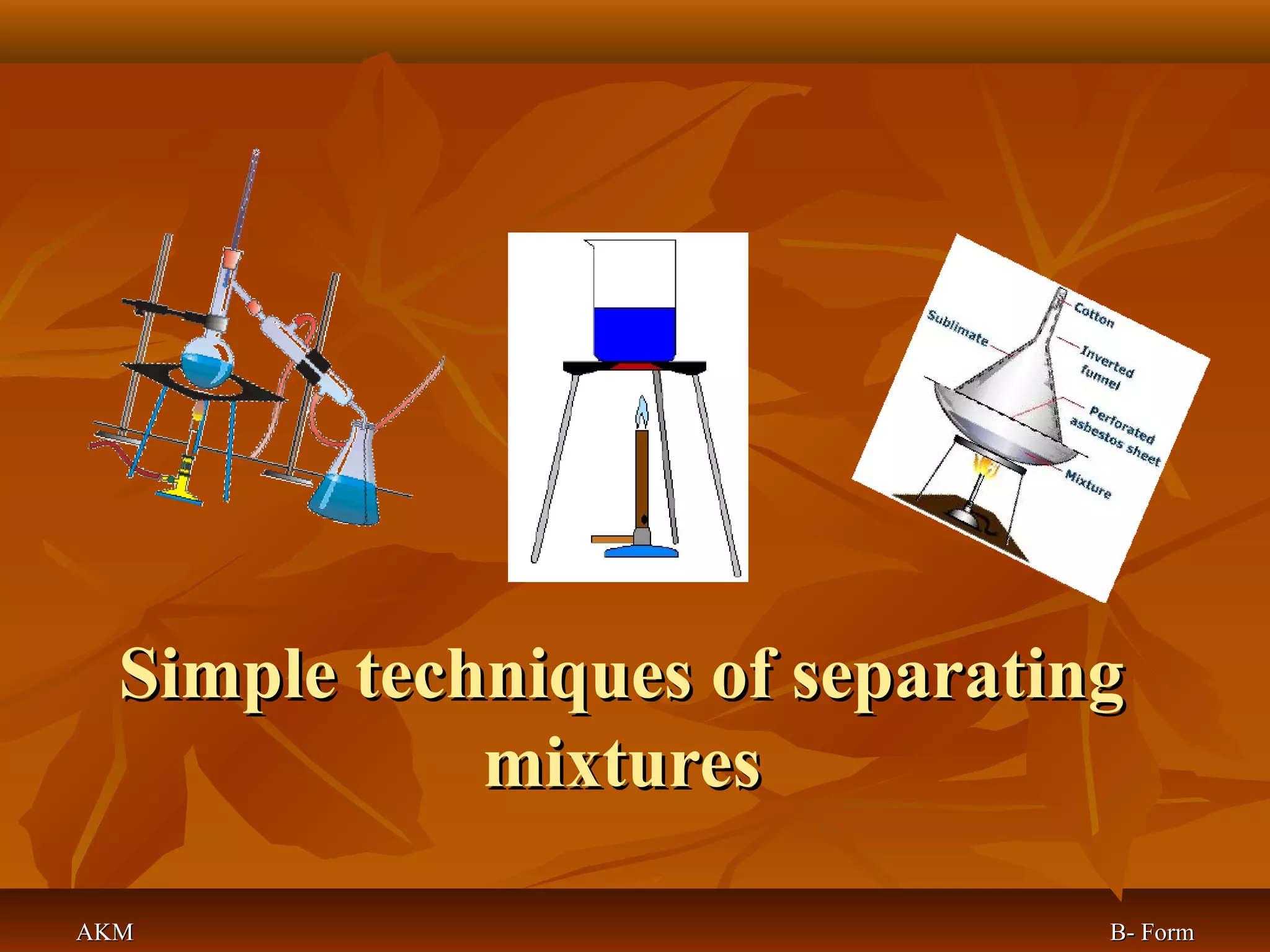
1) The document discusses various techniques for separating mixtures, including sedimentation, filtration, evaporation, distillation, crystallization, sublimation, chromatography, and solvent extraction.
2) These techniques allow students to separate mixtures based on differences in properties of the components such as solubility, boiling point, and polarity.
3) The lesson explains key terms and shows examples of separating mixtures of substances like sand and water, sulfur and water, salt and chalk using different techniques.

![AudienceAudience
Grade: 9Grade: 9
Age Group: 15 yearsAge Group: 15 years
Learner profiles –Different abilitiesLearner profiles –Different abilities
[intelligent, mediocre and slow learners][intelligent, mediocre and slow learners]
Their learning styles are differentTheir learning styles are different](https://image.slidesharecdn.com/simpletechniquesofseparatingmixtures-130803101929-phpapp01/75/Simple-techniques-of-separating-mixtures-3-2048.jpg)