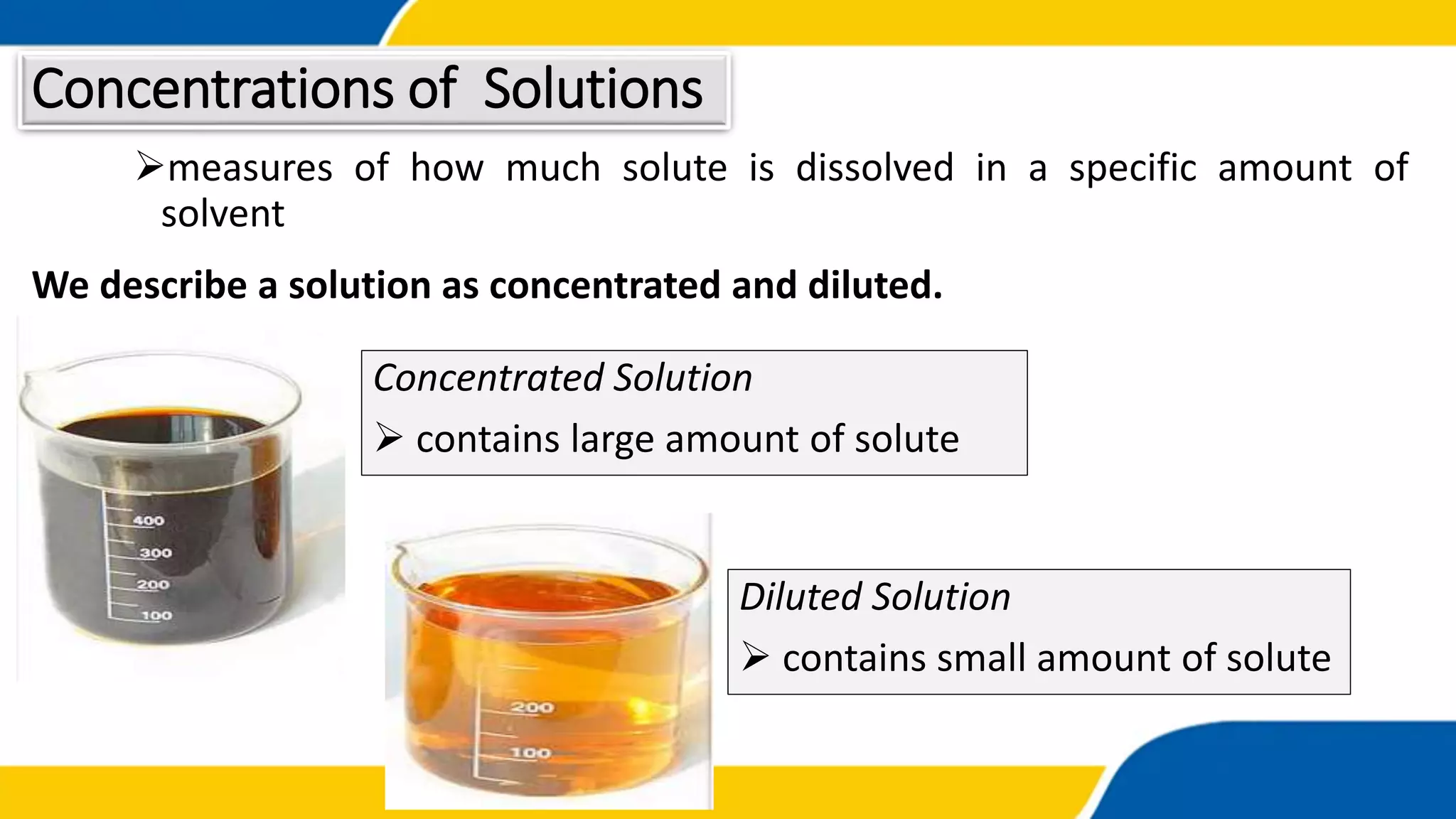
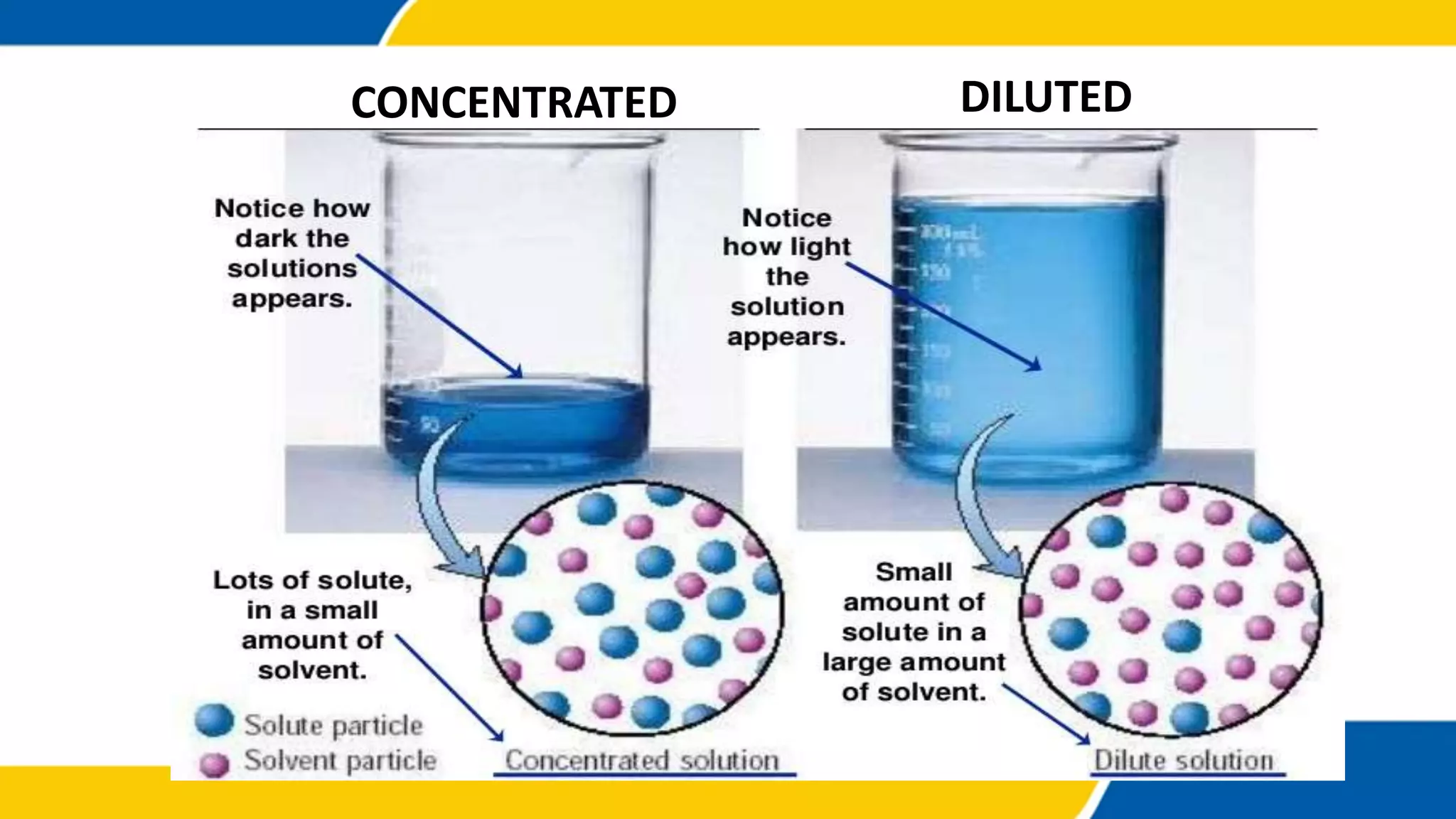
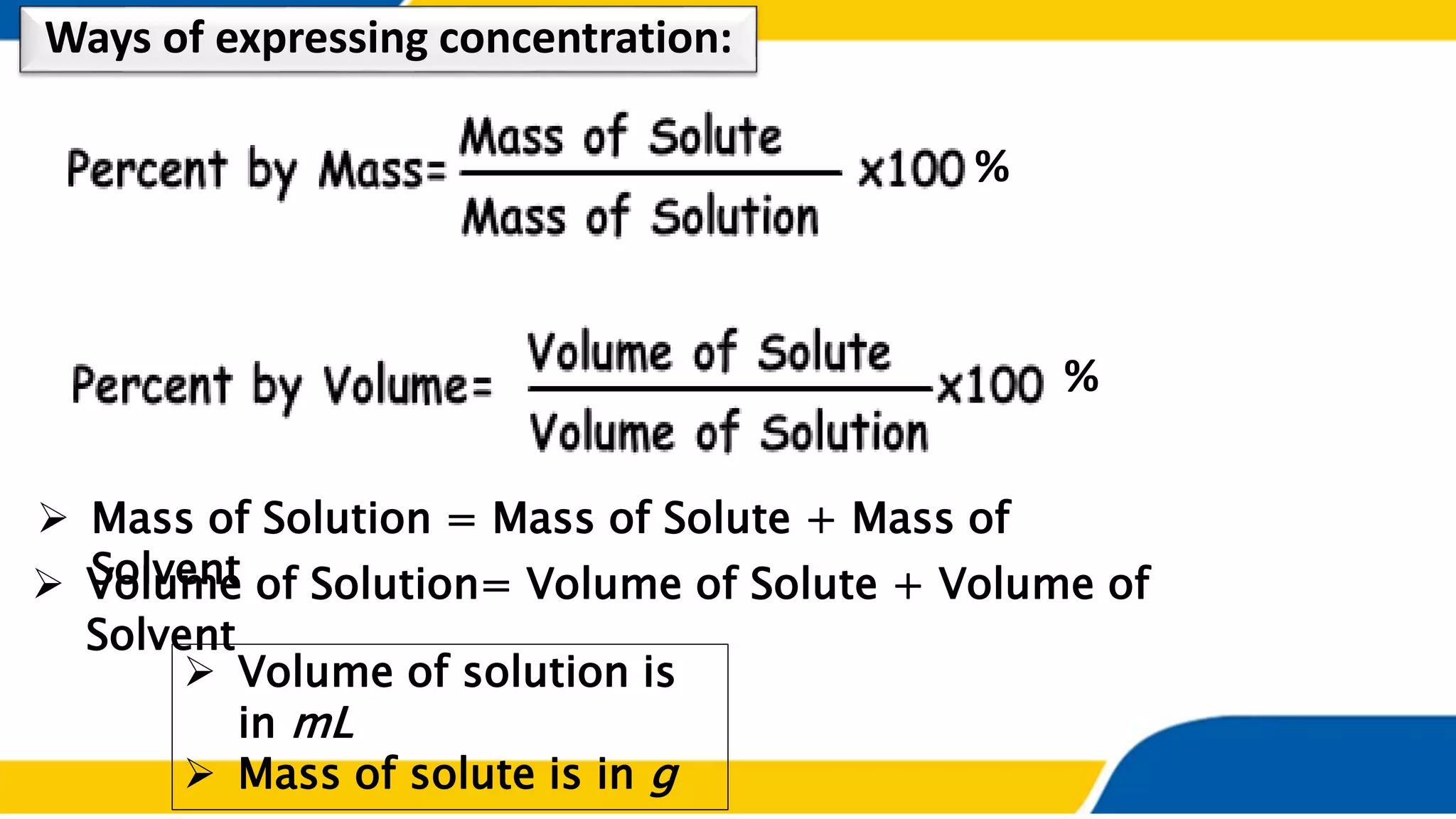
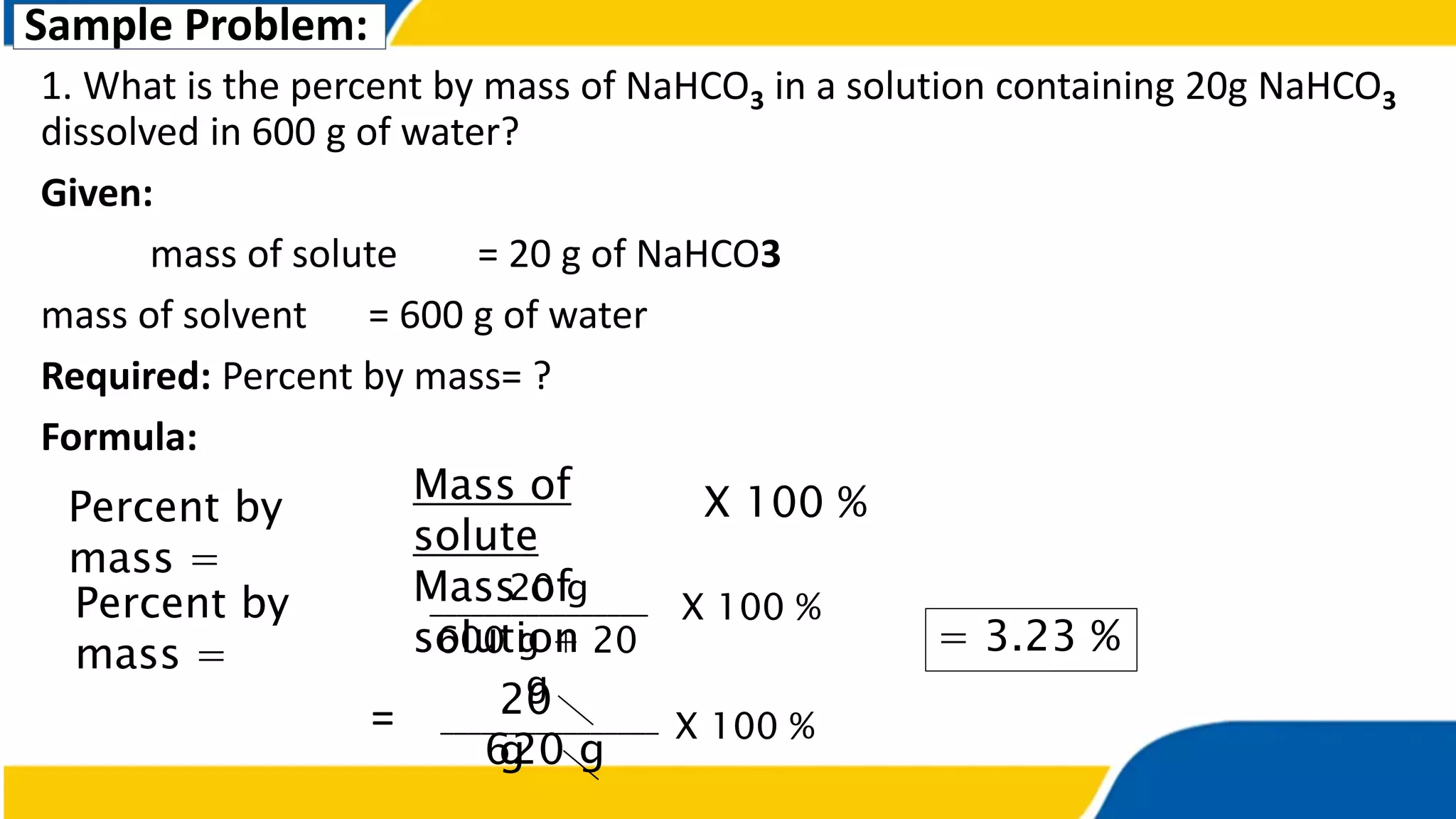
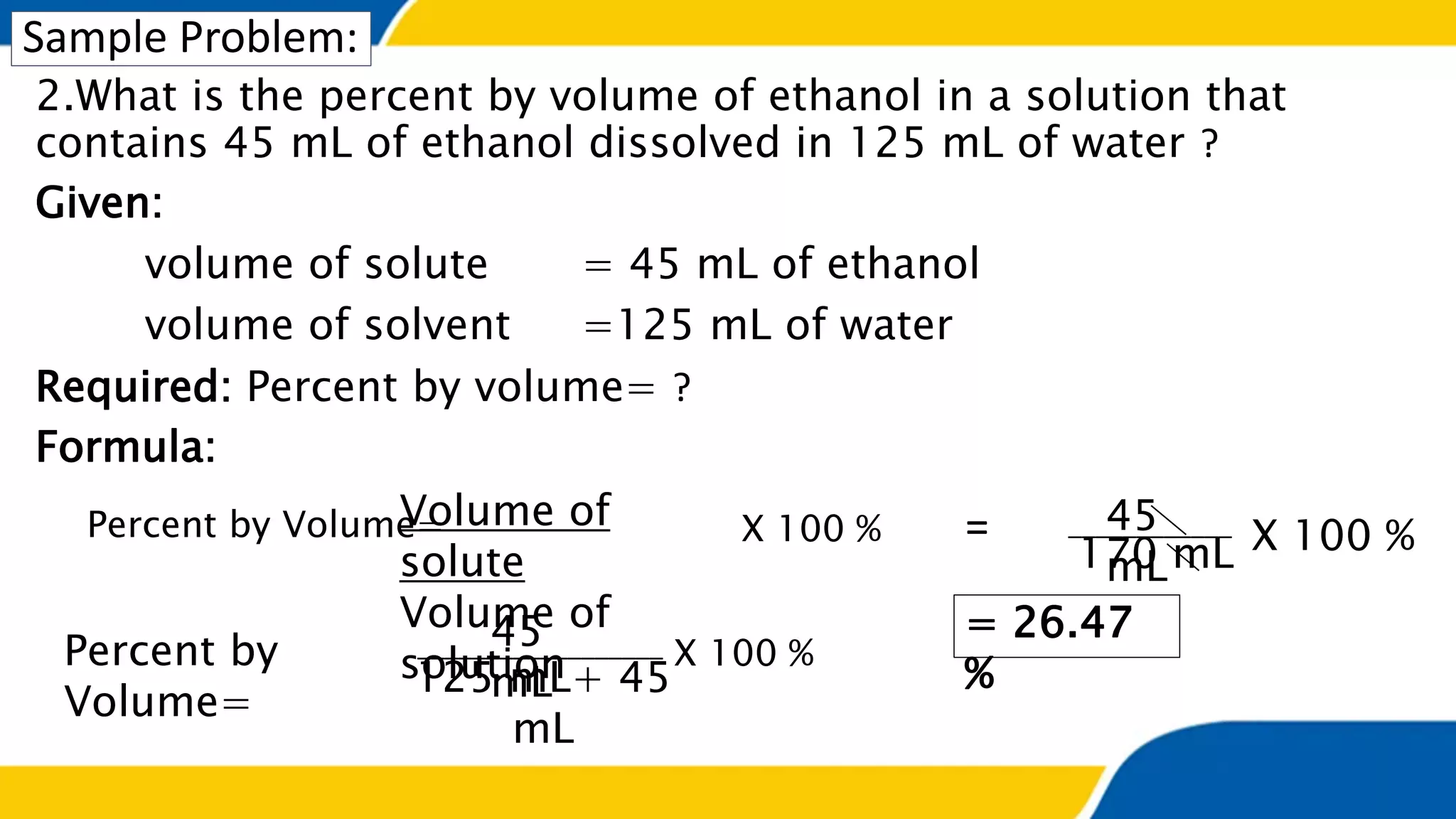
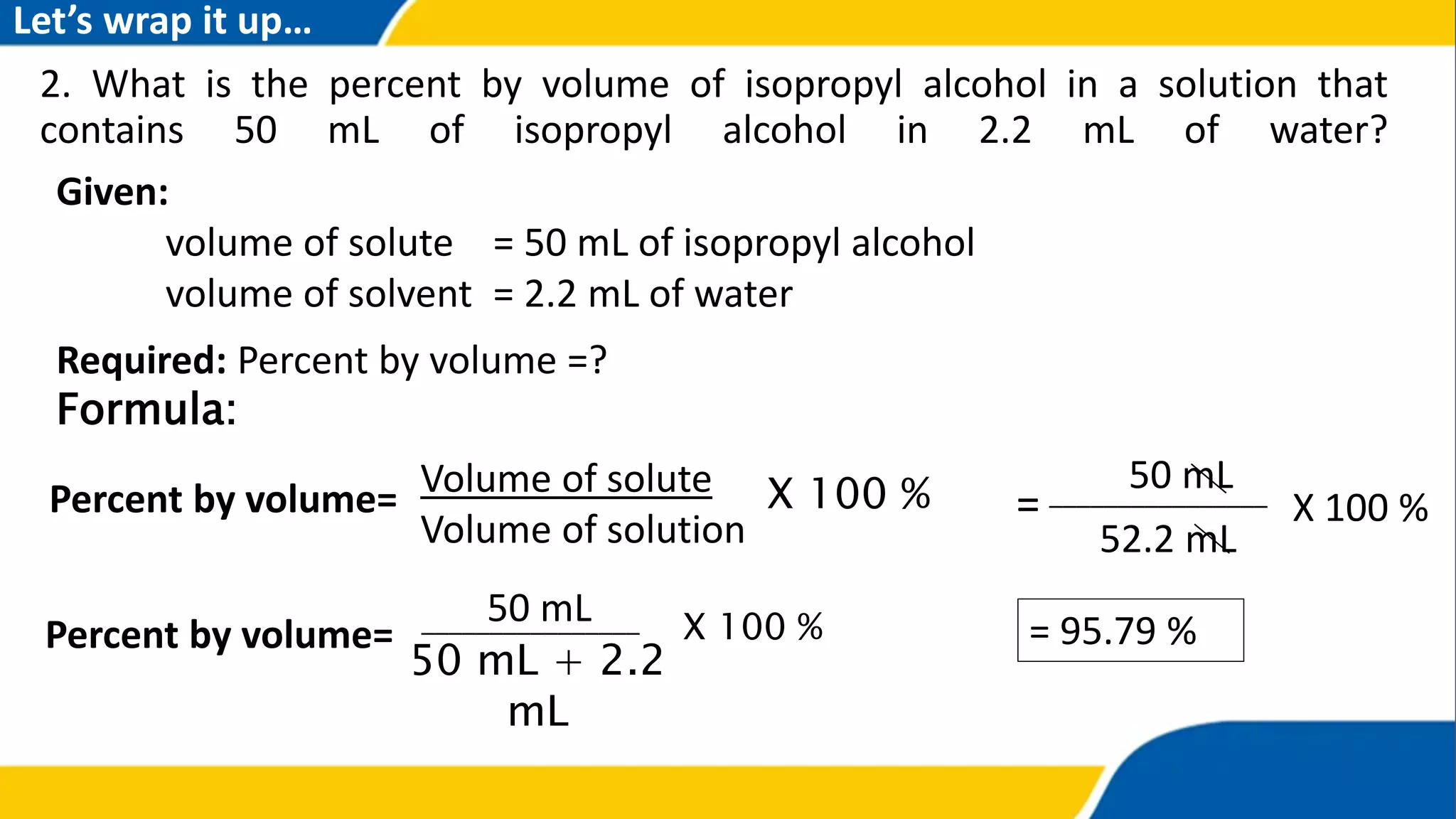
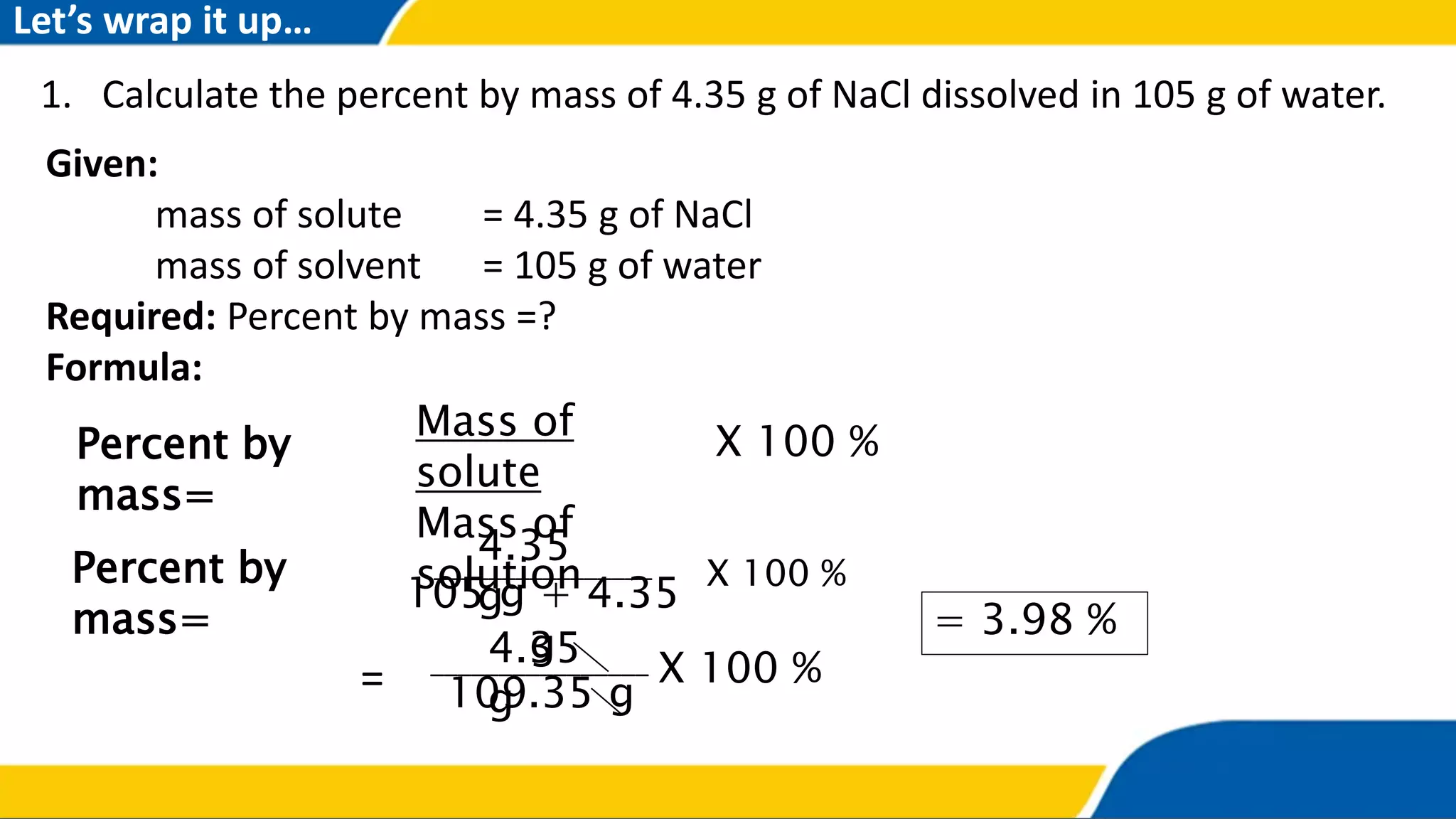The document outlines the objectives and concepts related to the concentration of solutions, including definitions of solute, solvent, and various types of solutions (concentrated, diluted, saturated, unsaturated, and supersaturated). It provides formulas for calculating percent by mass and percent by volume, along with sample problems to illustrate these calculations. Additionally, it includes instructions for practical activities and assessments to deepen understanding of the topic.