Embed presentation
Download to read offline


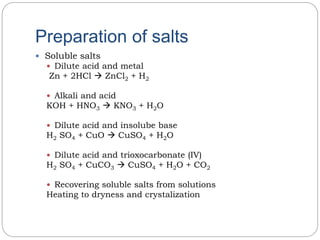

A salt is formed when all or part of the hydrogen in an acid is replaced by metal or ammonium ions. There are different types of salts including normal salts, acid salts, basic salts, double salts, and complex salts. Salts can be prepared through reactions such as mixing a dilute acid with a metal to form a soluble salt, reacting an alkali with an acid, and mixing solutions containing ions that form an insoluble salt to produce precipitation. Insoluble salts can also be prepared through double decomposition reactions or by directly combining element constituents.




