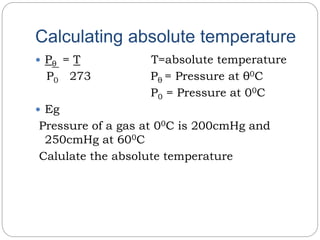
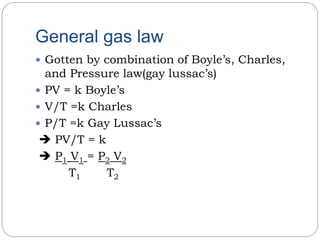
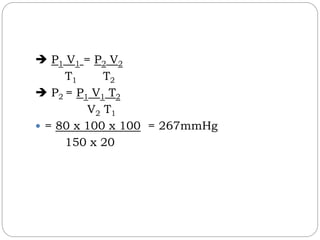
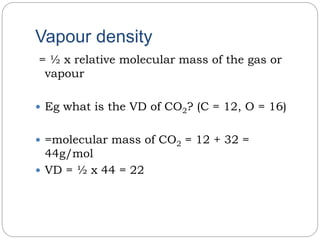
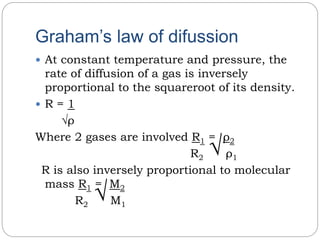
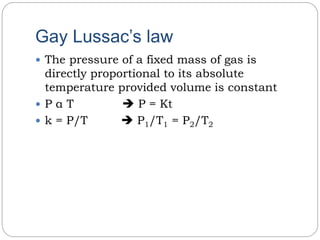The document summarizes several gas laws:
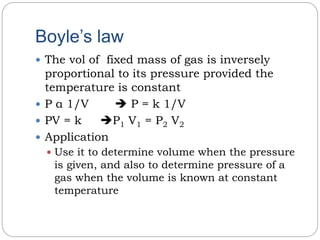
1) Boyle's law states that the volume of a fixed mass of gas is inversely proportional to its pressure at constant temperature.
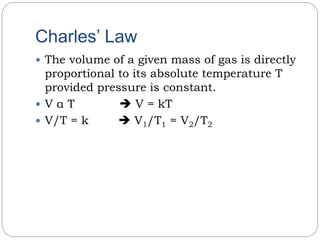
2) Charles' law states that the volume of a fixed mass of gas is directly proportional to its absolute temperature if pressure remains constant.
3) Gay-Lussac's law states that the pressure of a fixed mass of gas is directly proportional to its absolute temperature if volume remains constant.
4) The general gas law combines Boyle's, Charles', and Gay-Lussac's laws to relate the pressure, volume, temperature, and amount of gas in a system.