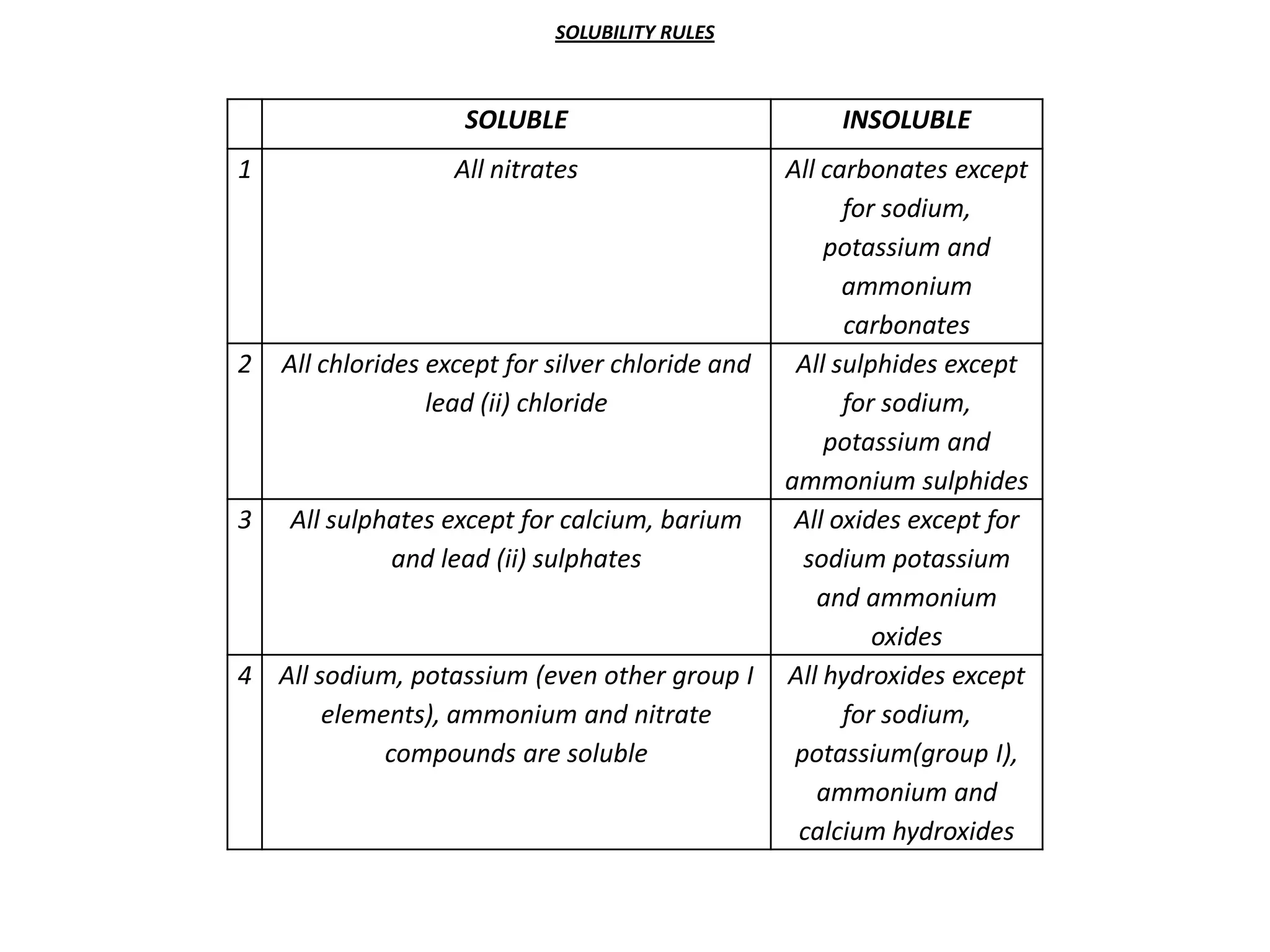
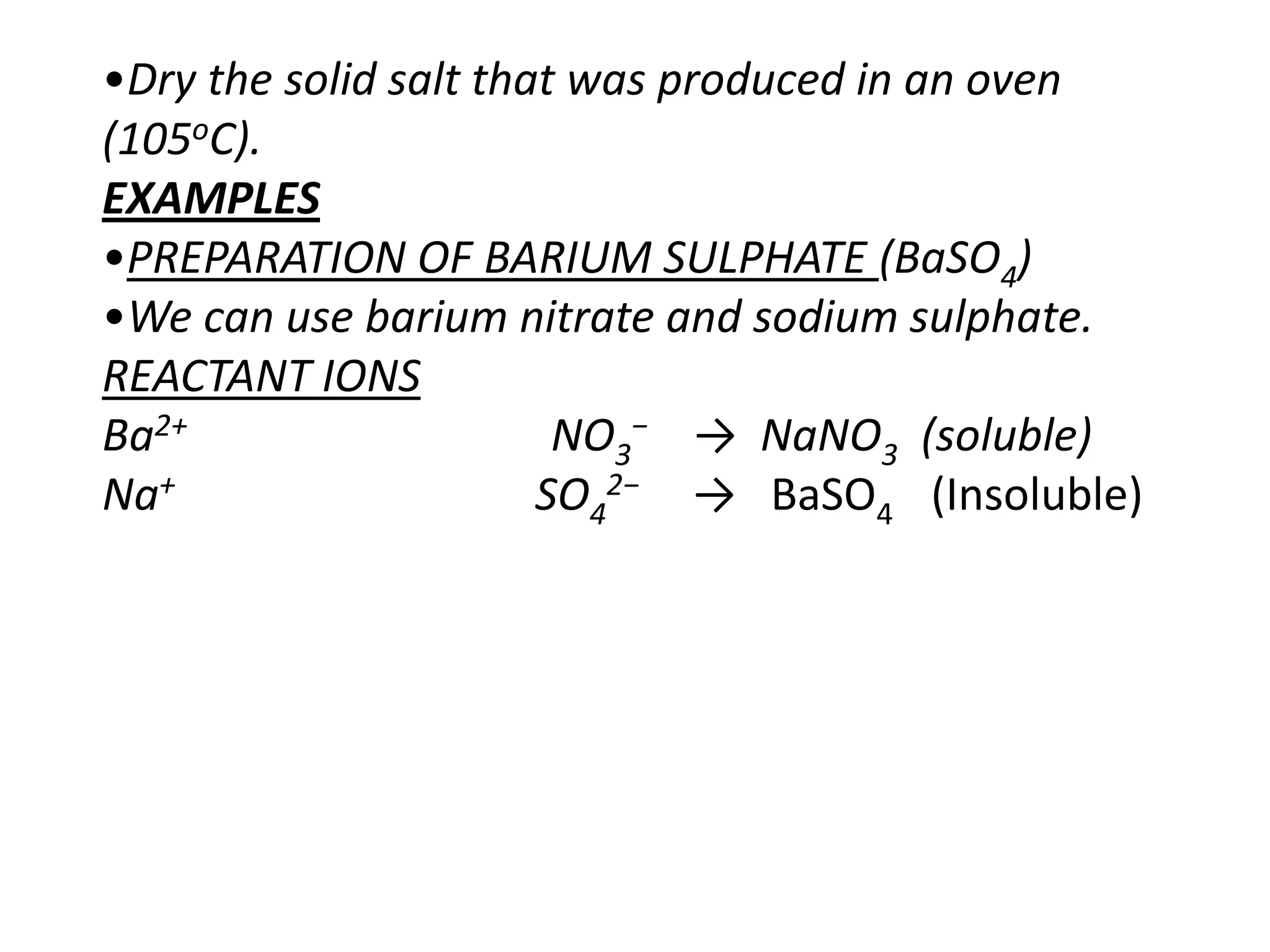
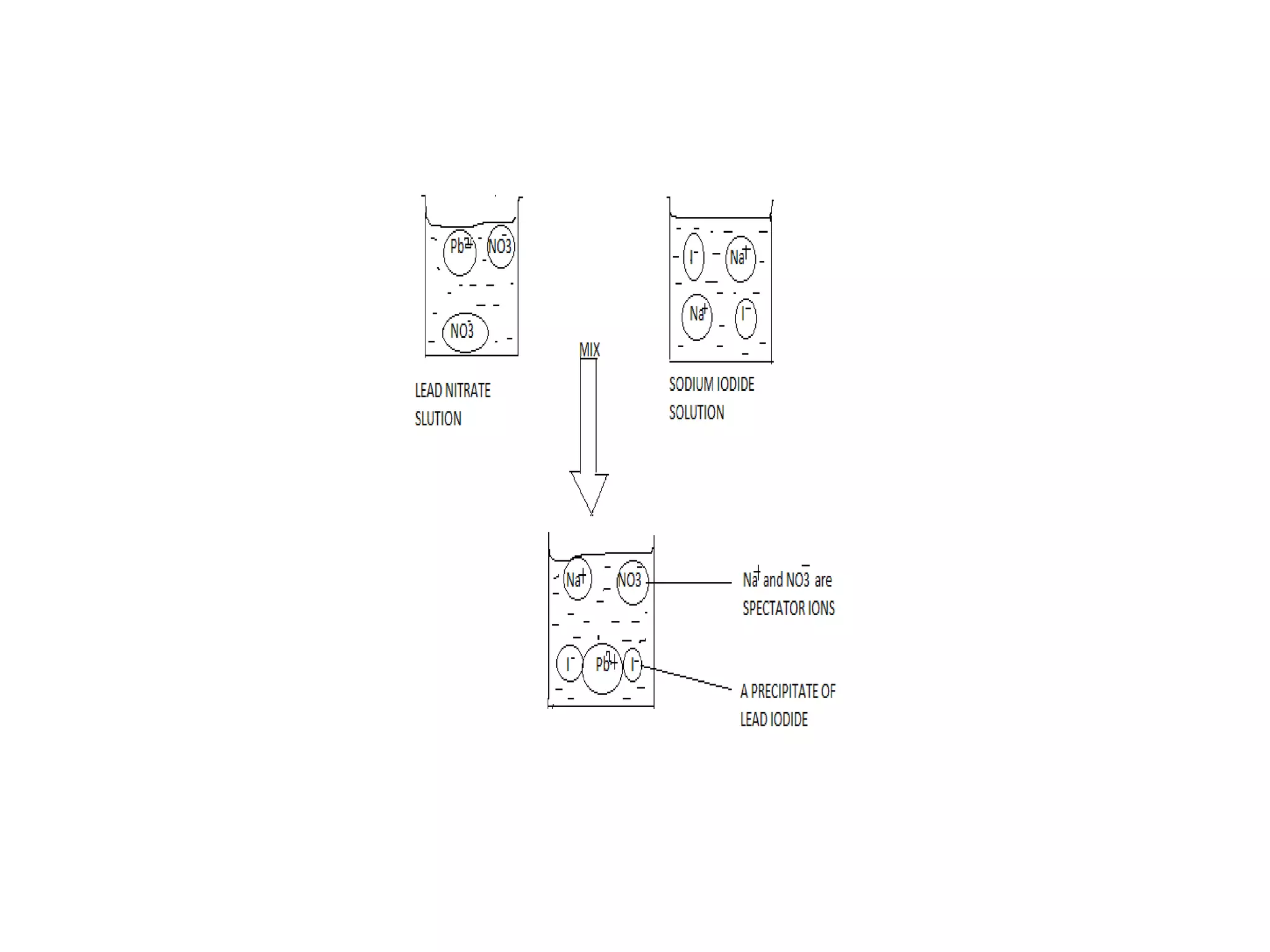
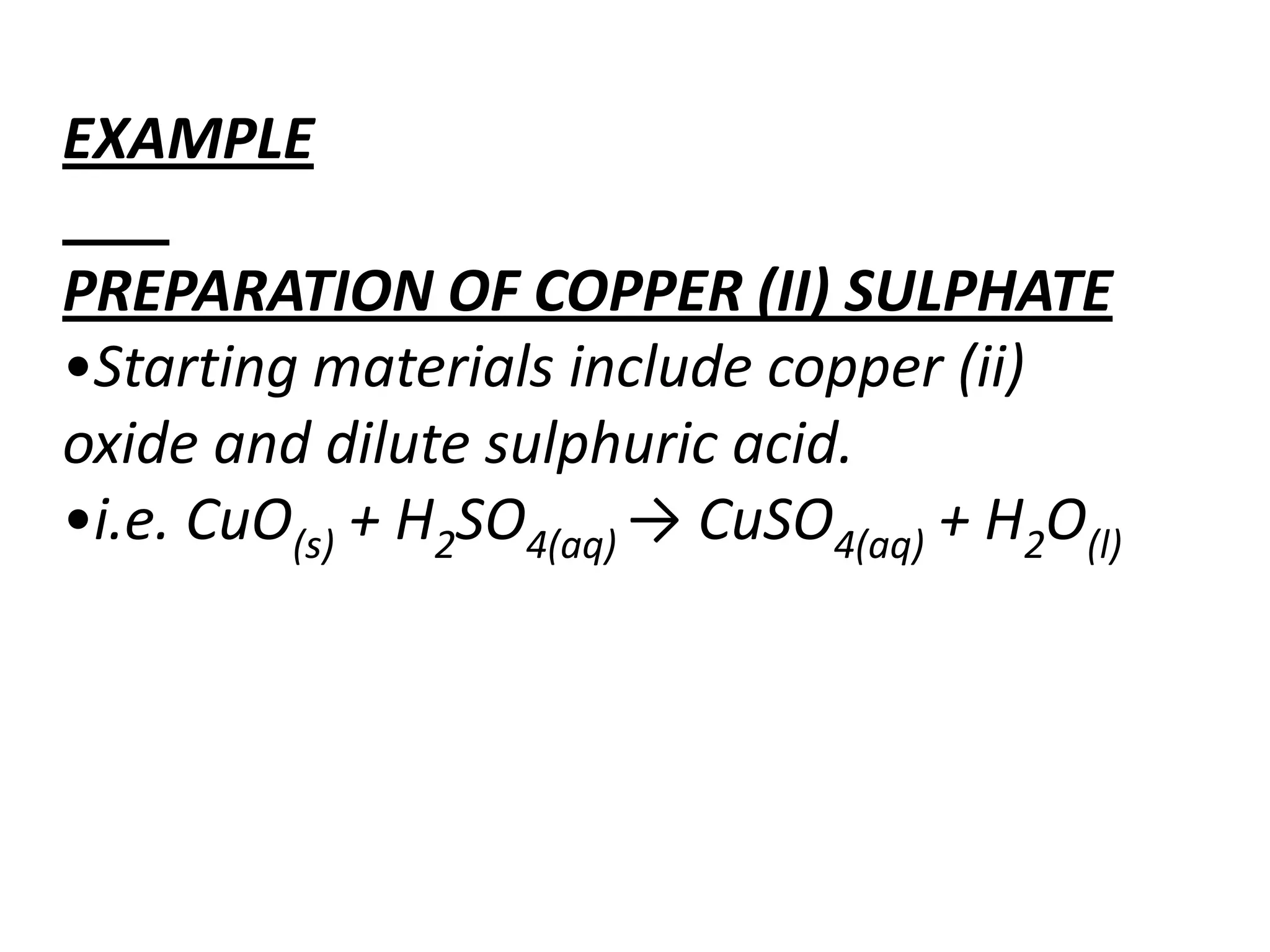
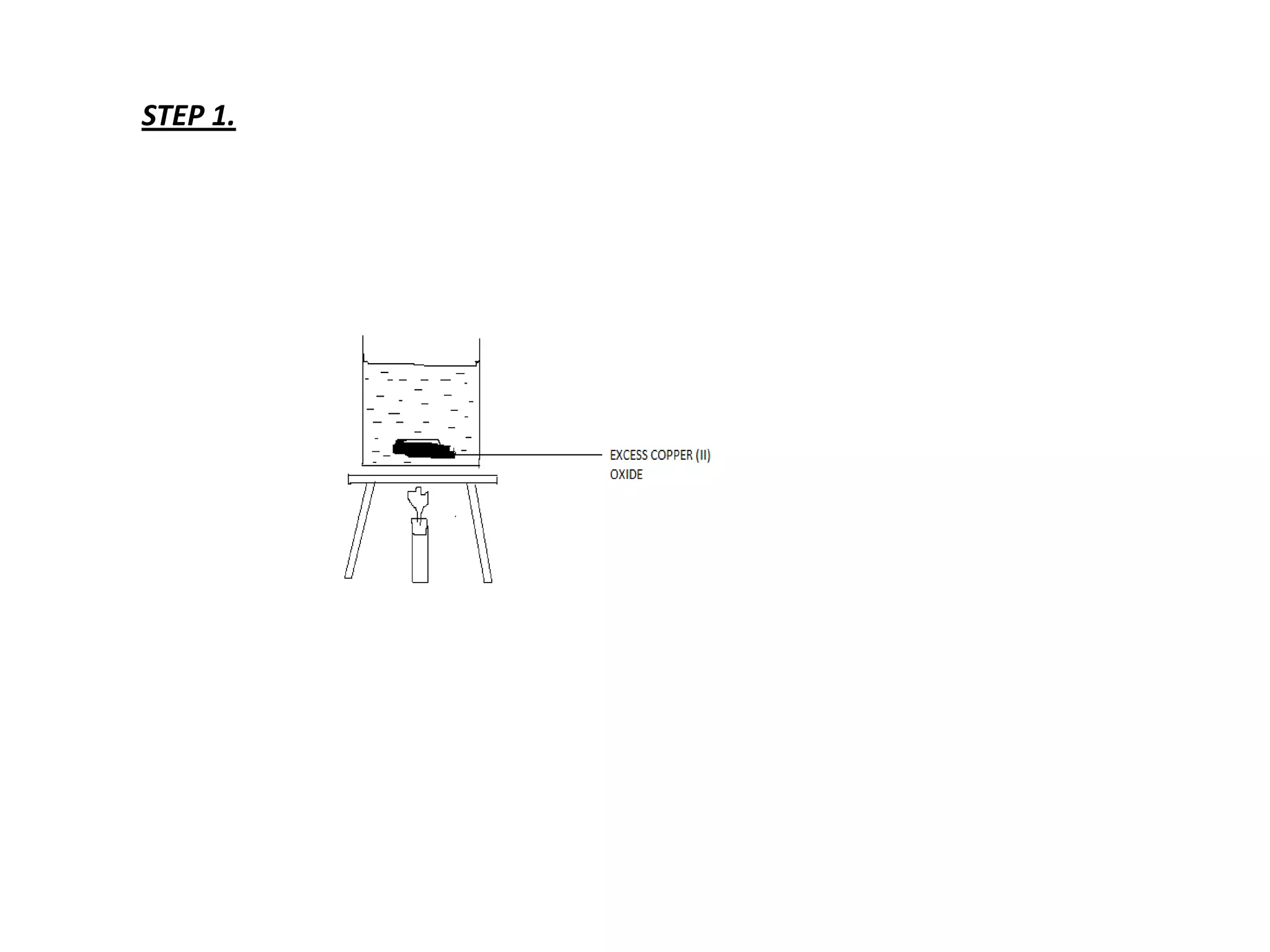
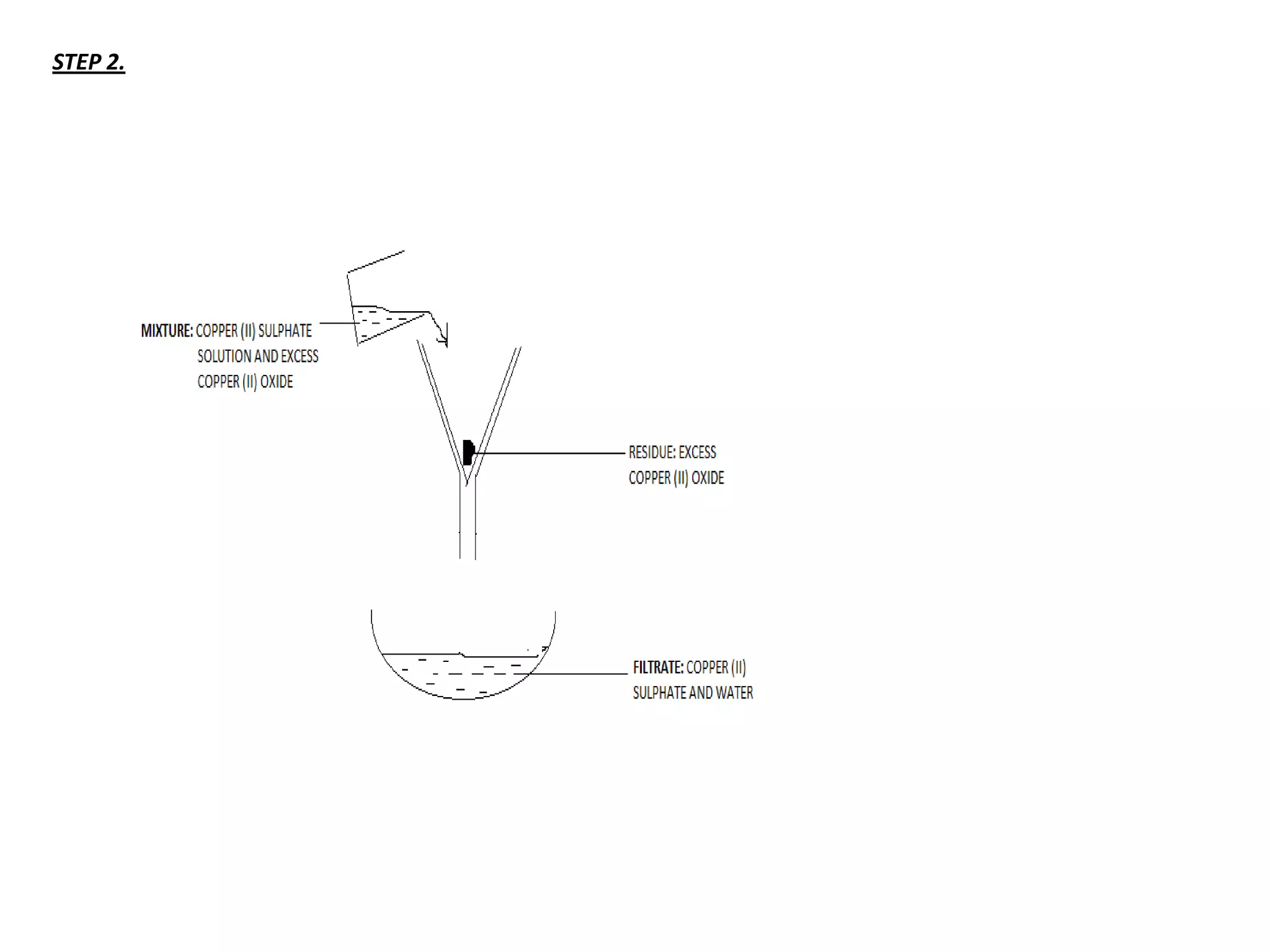
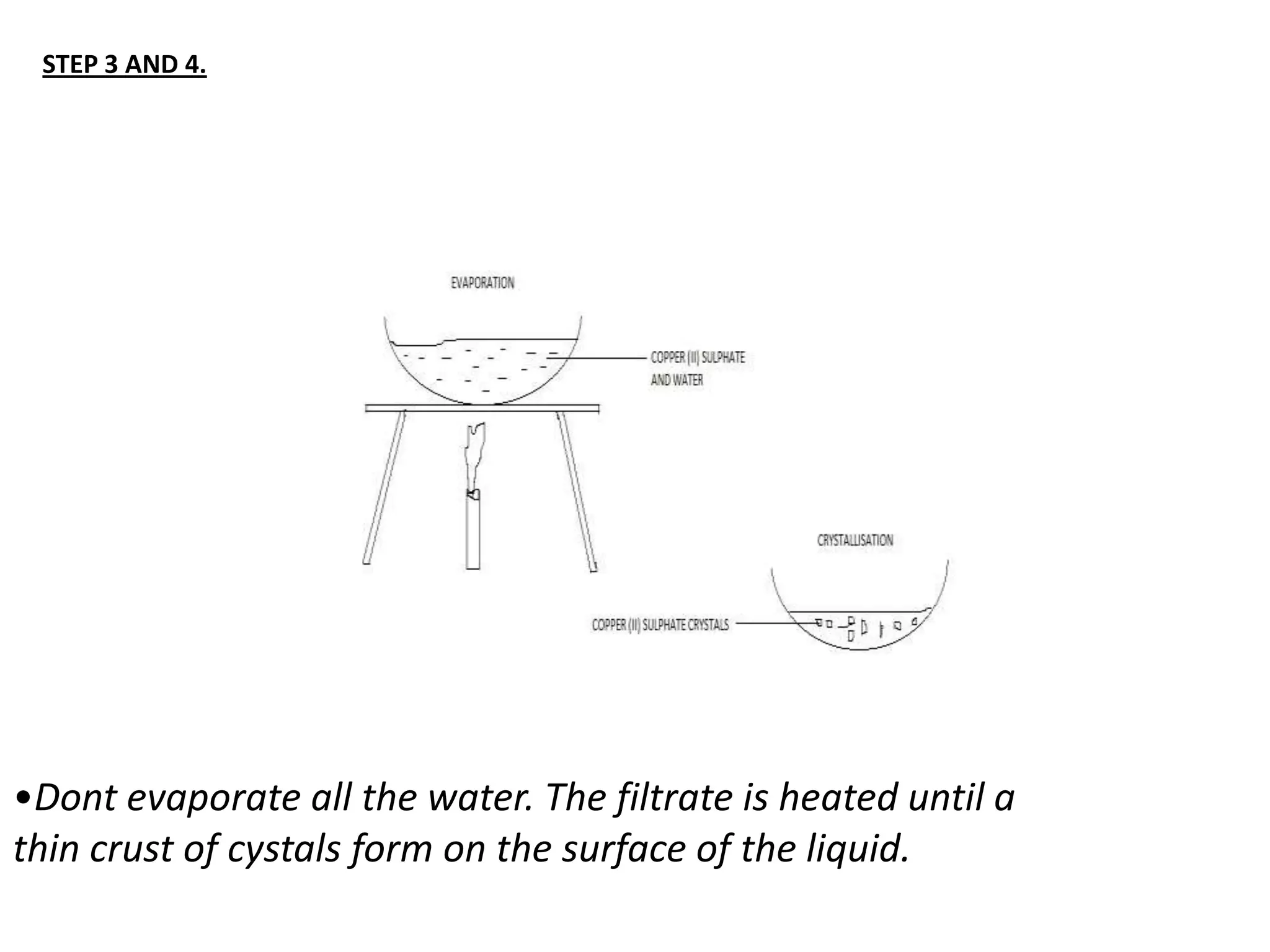
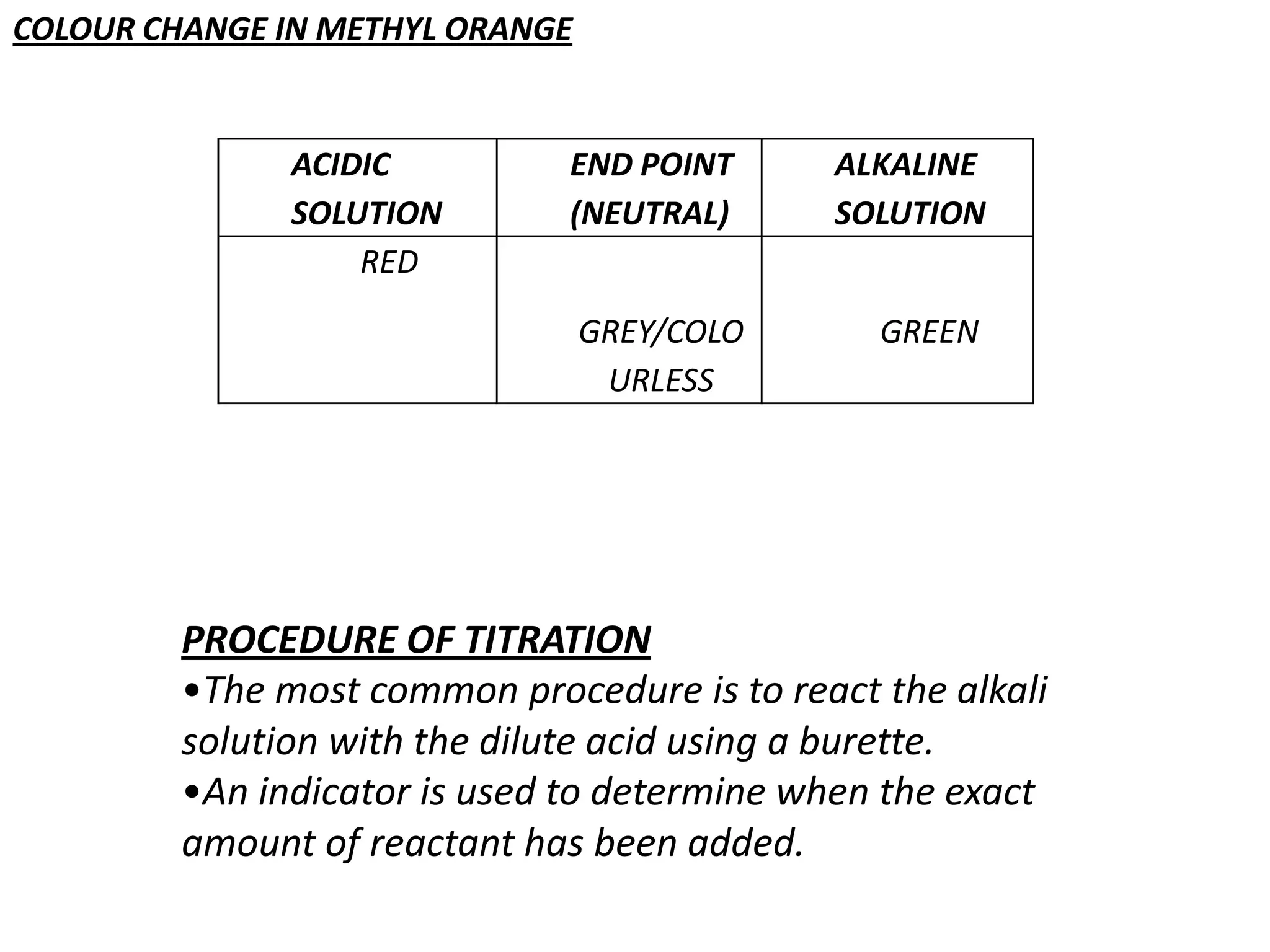
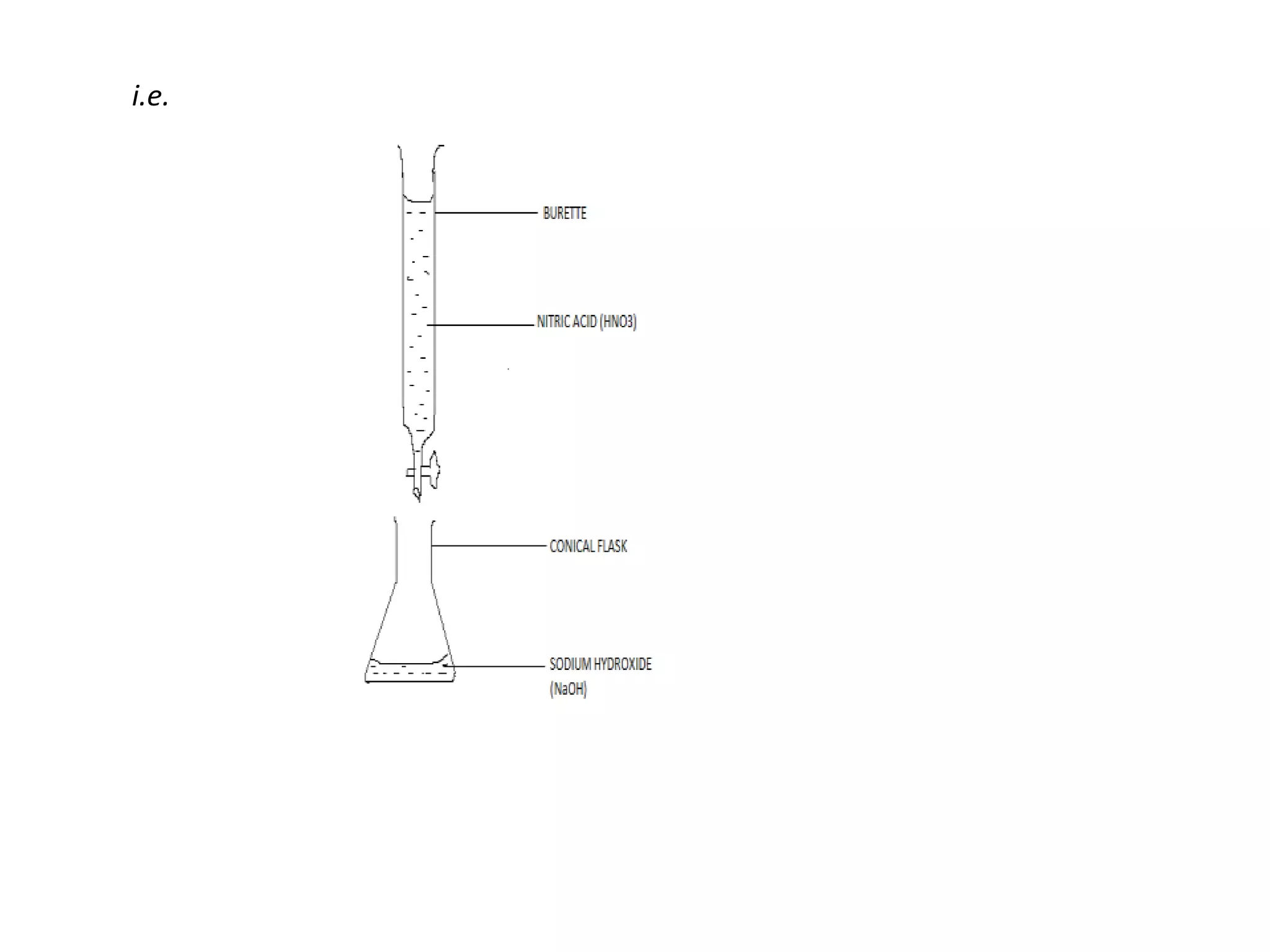
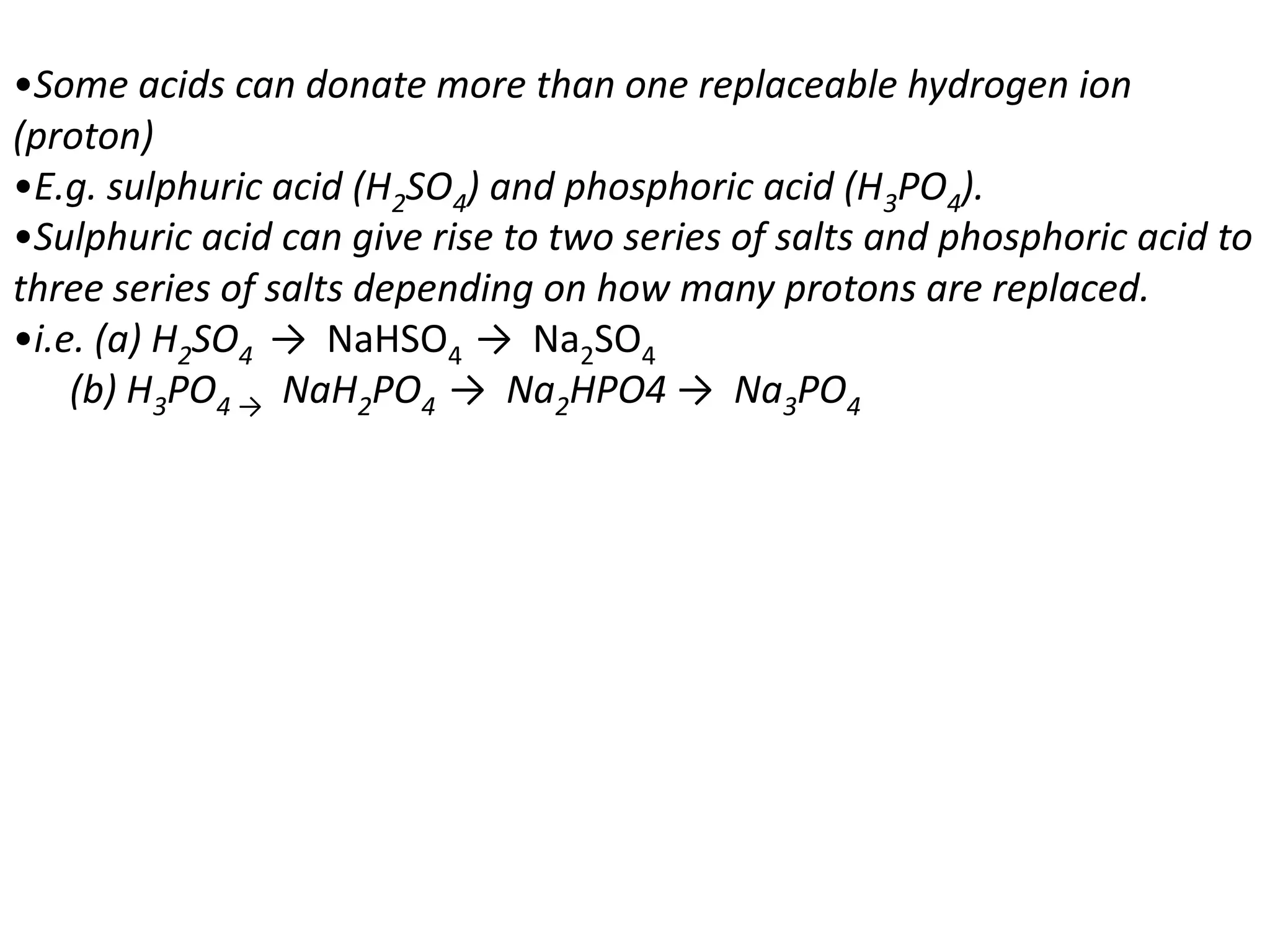Salts are formed when hydrogen ions in acids are replaced by metal ions or ammonium ions. There are two main types of salts - normal salts that do not contain replaceable hydrogen and acidic salts that contain replaceable hydrogen. Insoluble salts are prepared through precipitation reactions by mixing solutions of reactants containing the ions of the insoluble salt. Soluble salts can be prepared through filtration and crystallization using excess insoluble reactants or through titration using exact quantities of reactants.