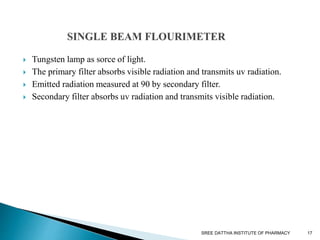
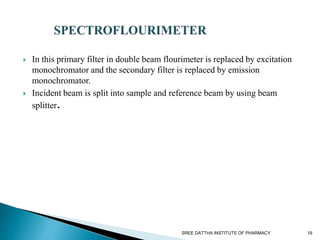
This document discusses fluorescence and fluorimetry. It begins with an introduction and overview of fluorescence as the emission of radiation when molecules are excited by radiation at certain wavelengths. It then discusses fluorimetry as the measurement of fluorescence intensity at a particular wavelength using a fluorimeter or spectrofluorimeter. Finally, it covers factors influencing fluorescence intensity, types of quenching, instrumentation components, and applications of fluorimetry in pharmaceutical analysis.





![SREE DATTHA INSTITUTE OF PHARMACY 15
PHOTO MULTIPLIER TUBE
The principle employed in this detector is that multiplication of photo
electrons by secondary emission of electrons.
This is achieved by using a photo cathode and a series of anodes
[dyanodes].upto 10 dyanodes are used.](https://image.slidesharecdn.com/salma-200414130858/85/Salma-15-320.jpg)