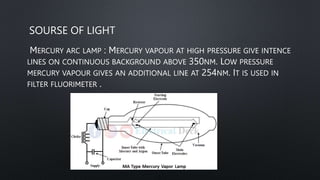
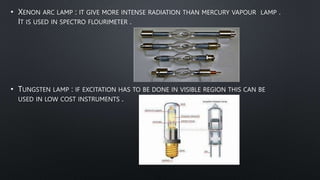
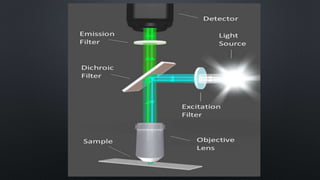
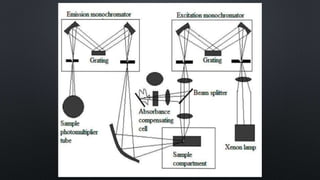
The document presents an overview of fluorimetry, covering its definitions, theories, factors affecting fluorescence, instrumentation, and applications. It explains fluorescence as the emission of light by substances upon exposure to light, distinguishing it from phosphorescence, which continues after the light source is removed. Key components discussed include light sources, detectors, and the significance of factors like temperature and pH on fluorescence intensity.










![SELF QUENCHING OR CONCENTRATION QUENCHING
AT LOW CONCENTRATIONS [ΜG OR NG/ML] , LINEARITY IS OBSERVED . AT HIGH
CONCENTRATIONS [MG/ML] OF THE SAME SUBSTANCE , PROPORTIONATE INCREASE IN
FLUORESCENCE INTENSITY DOES NOT OCCUR . THIS PHENOMENON IS CALLED AS SELF
QUENCHING OR CONCENTRATION QUENCHING .
CHEMICAL QUENCHING : IN THIS TYPE , QUENCHING IS DUE TO FACTORS LIKE CHANGE IN
PH , PRESENCE OF OXYGEN , HALIDES OR HEAVY METALS .](https://image.slidesharecdn.com/shivampat11-230309054734-7365e24b/85/FLOURIMETRY-pptx-11-320.jpg)