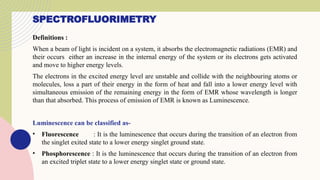
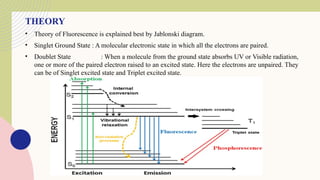
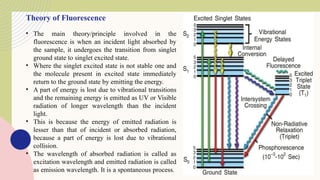
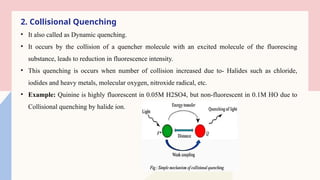
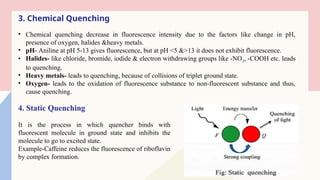
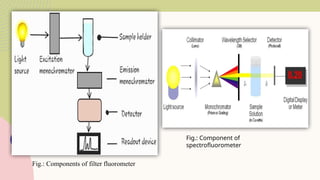
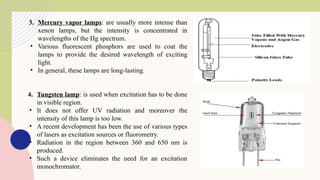
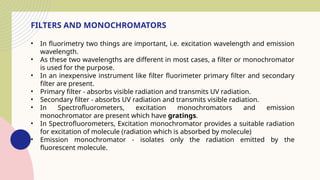
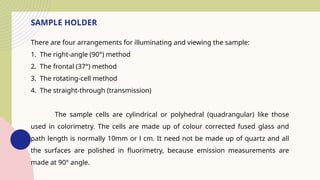
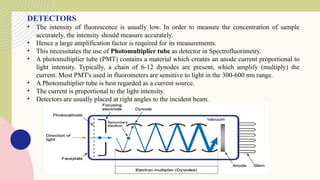
Spectrofluorimetry is an analytical technique used to detect and quantify substances based on their ability to emit light (fluorescence) after being excited by a specific wavelength of light. When a compound absorbs light, it moves to an excited state and then emits light as it returns to its ground state. This emitted light is measured to determine the concentration of the compound.
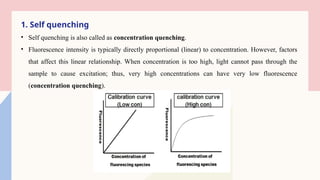
The technique is highly sensitive and selective, making it suitable for detecting very low levels of substances, especially in pharmaceutical, clinical, environmental, and food analysis. However, it is limited to compounds that naturally fluoresce or can be made fluorescent, and its accuracy can be affected by factors like pH, temperature, and the presence of quenching agents.