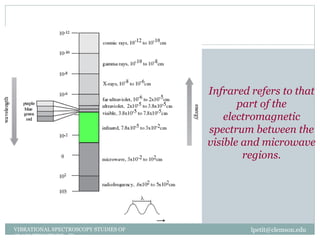
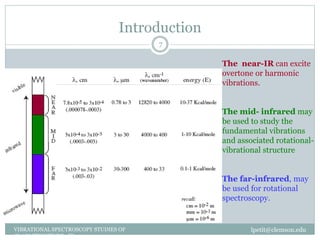
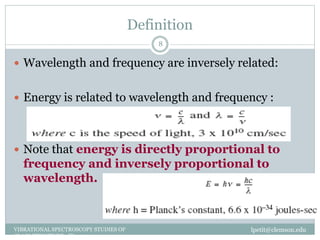
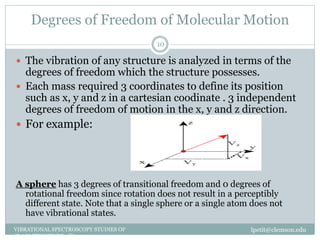
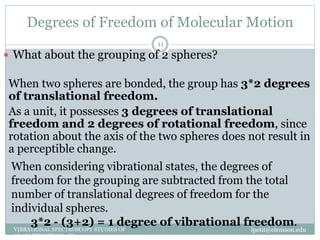
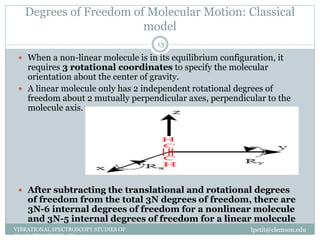
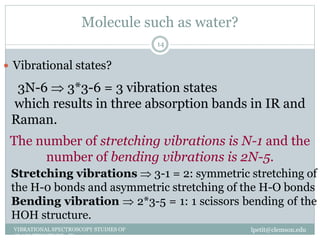
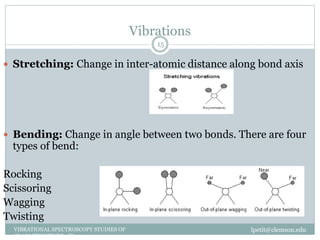
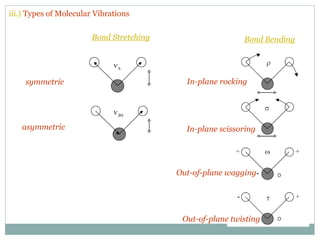
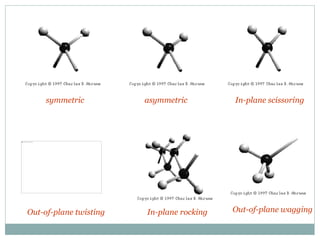
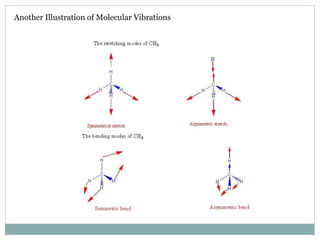
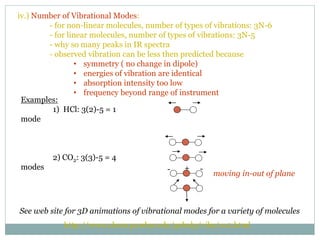
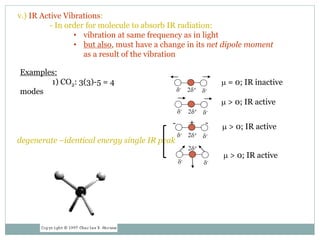
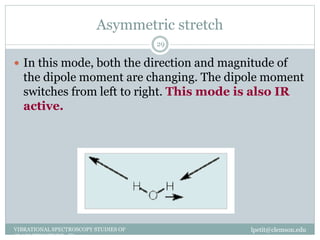
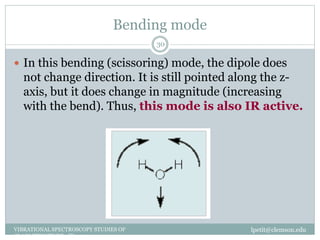
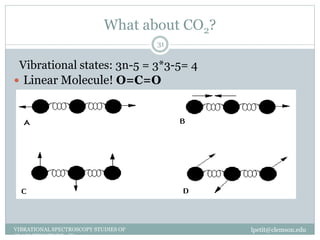
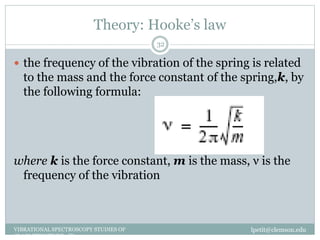
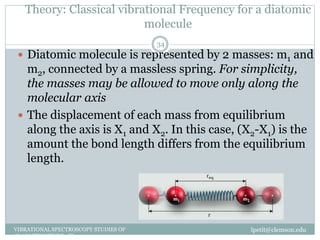
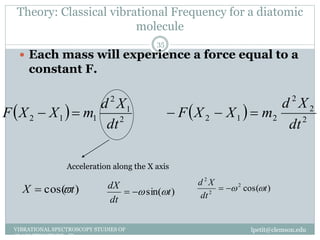
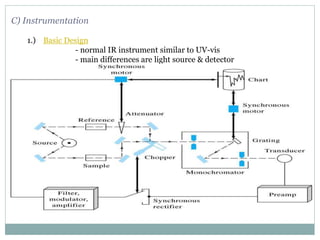
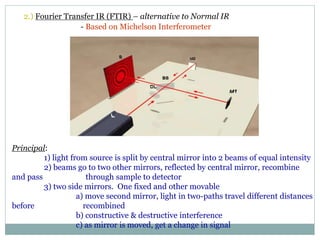
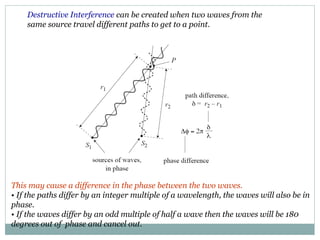
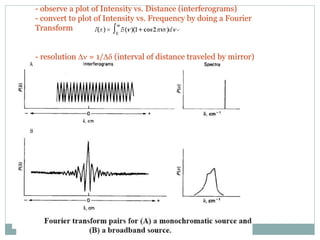
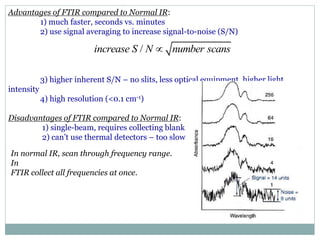
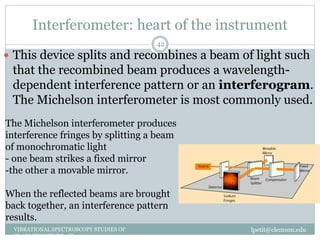
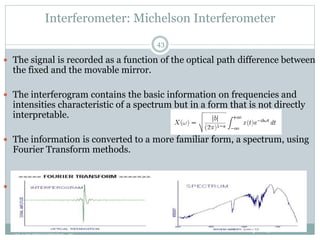
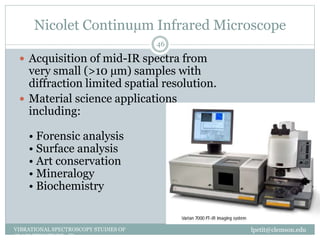
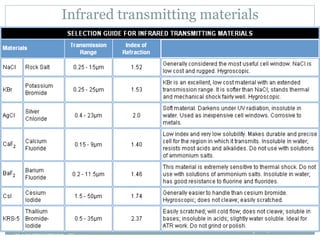
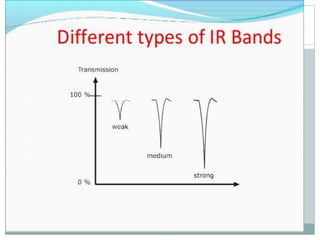
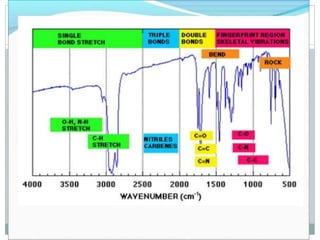
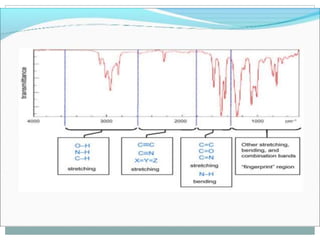
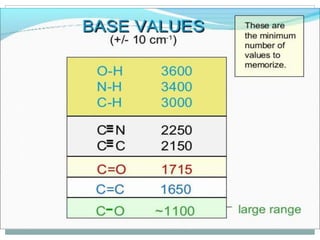
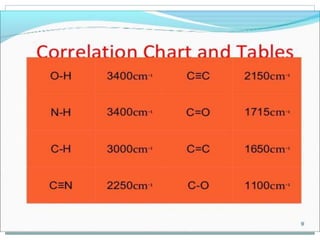
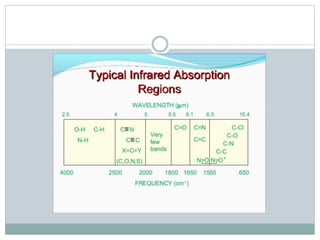
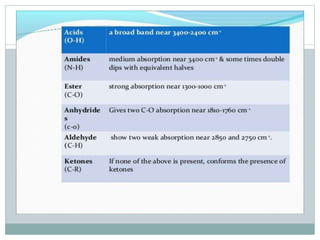
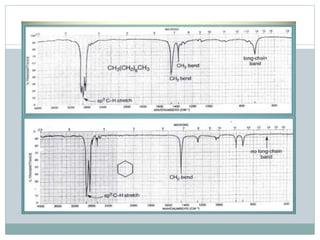
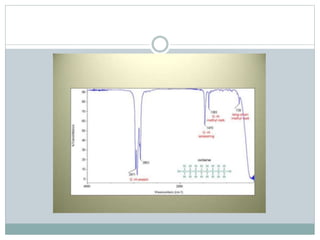
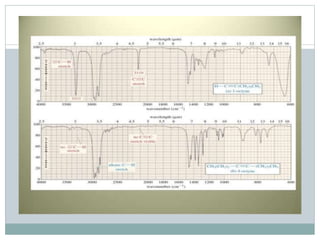
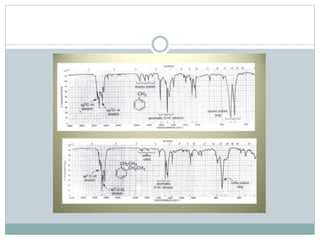
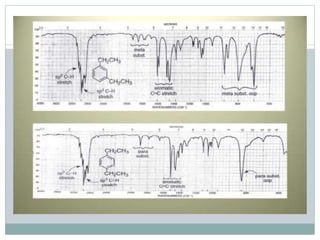
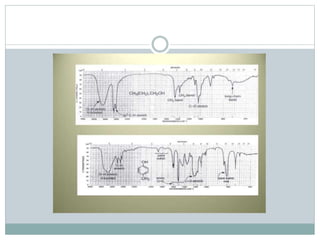
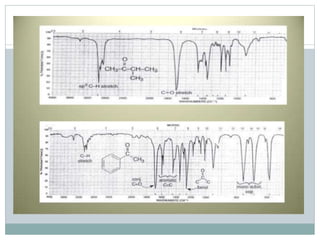
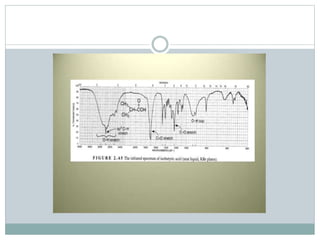
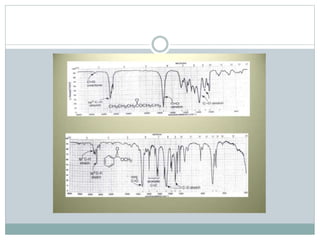
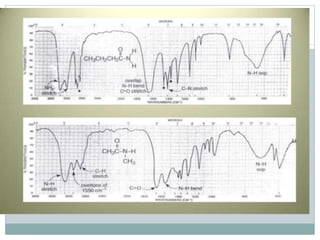
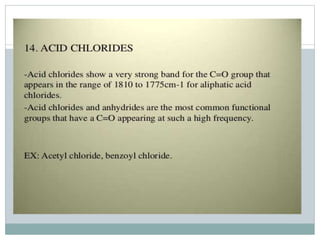
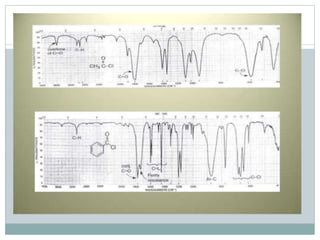
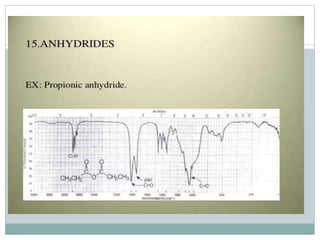
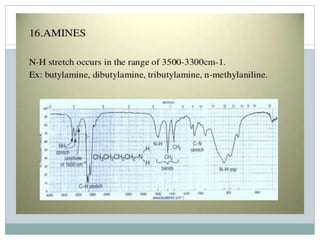
This document discusses infrared (IR) spectroscopy and how it can be used to analyze molecular vibrations. It explains that IR spectroscopy measures the absorption of IR radiation by materials as their atoms vibrate in different ways. Molecules absorb IR radiation at frequencies related to their unique compositions, structures, and bond types. The number and types of vibrational modes a molecule can undergo depends on the number of atoms and whether the molecule is linear or nonlinear. For a vibration to be IR active, it must involve a change in the molecule's dipole moment as it vibrates. Examples are provided of analyzing the vibrational modes and IR activity of molecules like water.