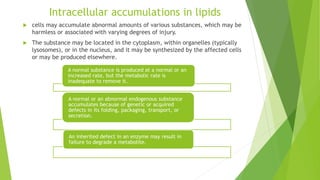
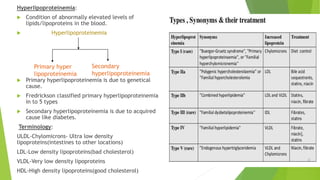
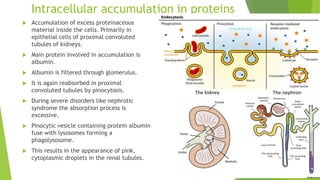
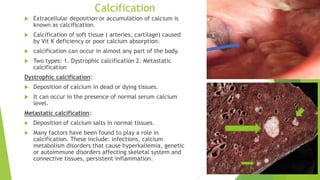
The document discusses various forms of cellular injury, including intracellular accumulations of lipids, proteins, carbohydrates, and calcification, leading to potential tissue damage. It covers hyperlipoproteinemia, enzyme leakage, alkalosis, acidosis, and electrolyte imbalances, detailing their causes, effects, and classifications. The information highlights the role of metabolism, genetic factors, and structural abnormalities in these conditions, along with their implications for human health.






![Electrolyte Imbalance:
The level of electrolyte in the body is abnormal called as electrolyte imbalance.
The most serious electrolyte disturbance involves abnormalities in the levels of
Sodium, Potassium or Calcium.
Causes: include diarrhea, vomiting, perspiration, injury, blood loss, fluid loss from
burns, eating disorders, alcoholism, cancer, diabetes and certain medication.
Malabsorption-The body may be unable to absorb these electrolytes due to a
variety of stomach disorders, medications.
Chemotherapy: Chemotherapy drugs (Cisplatin) Diuretics (furosemide [Lasix] or
Bumetanide) Antibiotics (Amphotericin B) ,Corticosteroids (Hydrocortisone).
Symptoms:
Blood test results indicate an altered potassium, magnesium, sodium, or calcium
levels, may experience muscle spasm, weakness, twitching, or convulsions.
Blood test results showing low sodium levels may lead to: irregular heartbeat,
confusion, blood pressure changes, nervous system or bone disorder.
Blood test results showing high levels of calcium may lead to: weakness or
twitching of the muscles, numbness, fatigue, and irregular heartbeat and blood
pressure changes.](https://image.slidesharecdn.com/cellinjurylecture-3-210424065118/85/Cell-injury-lecture-3-12-320.jpg)