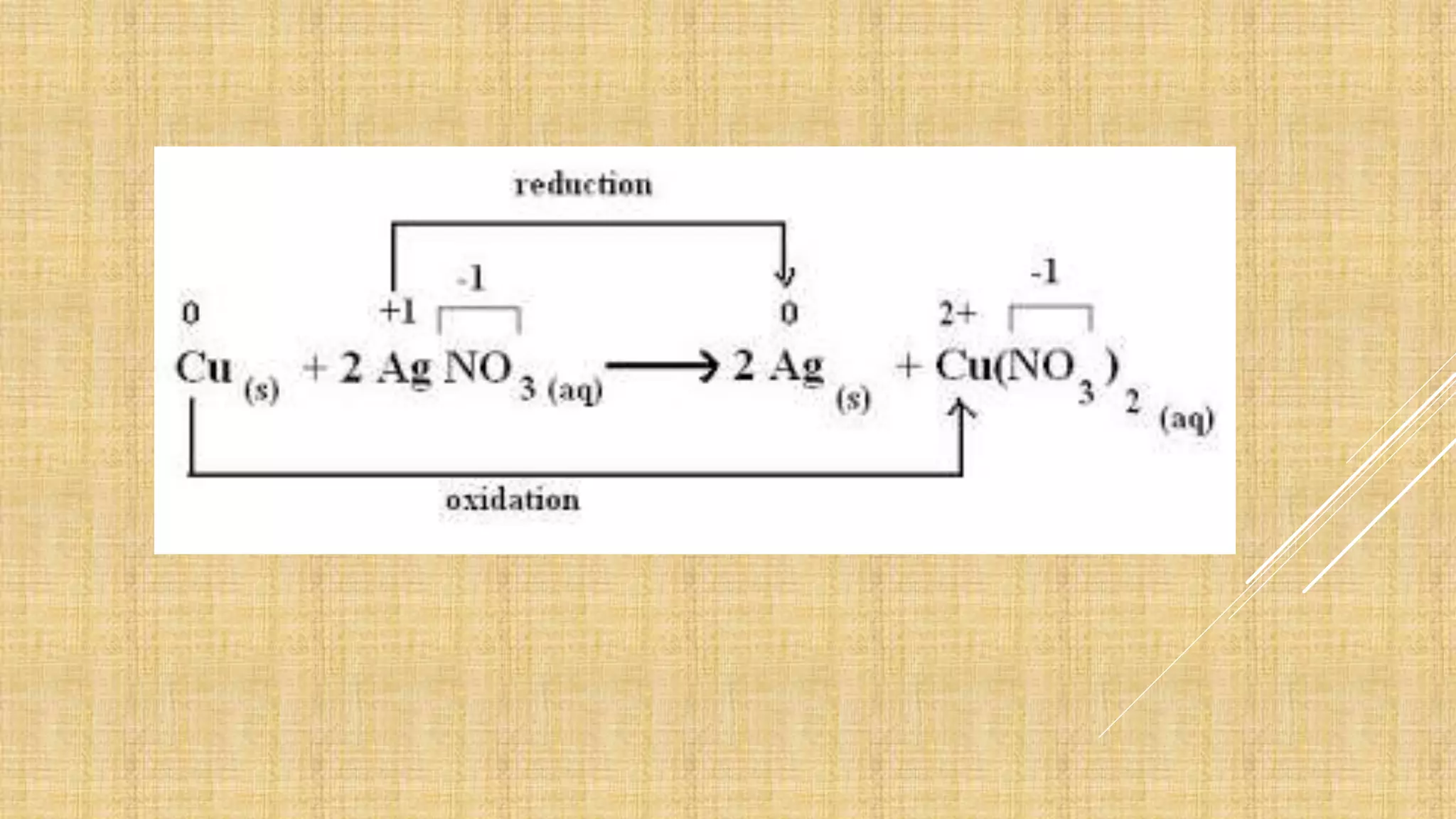
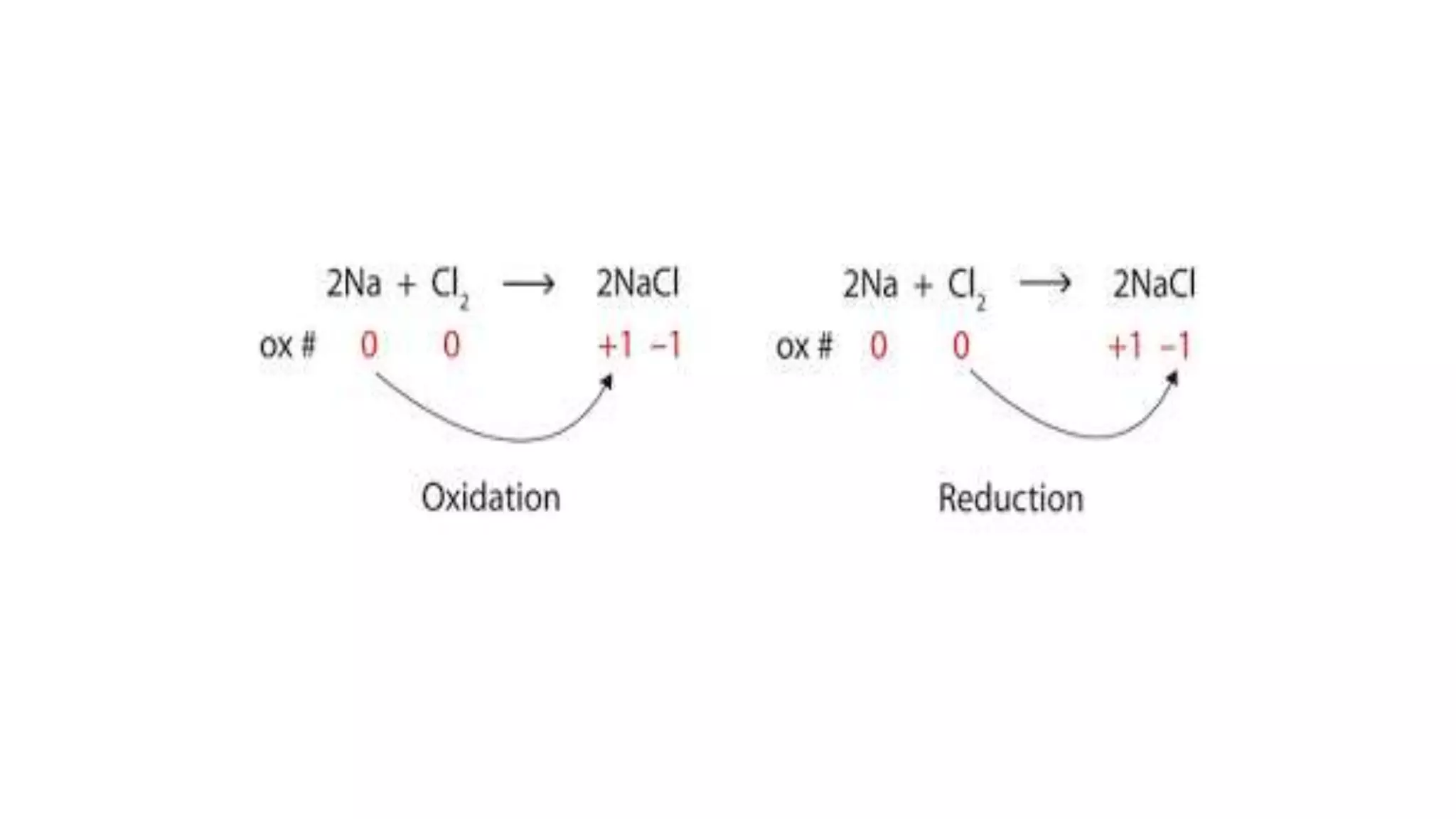
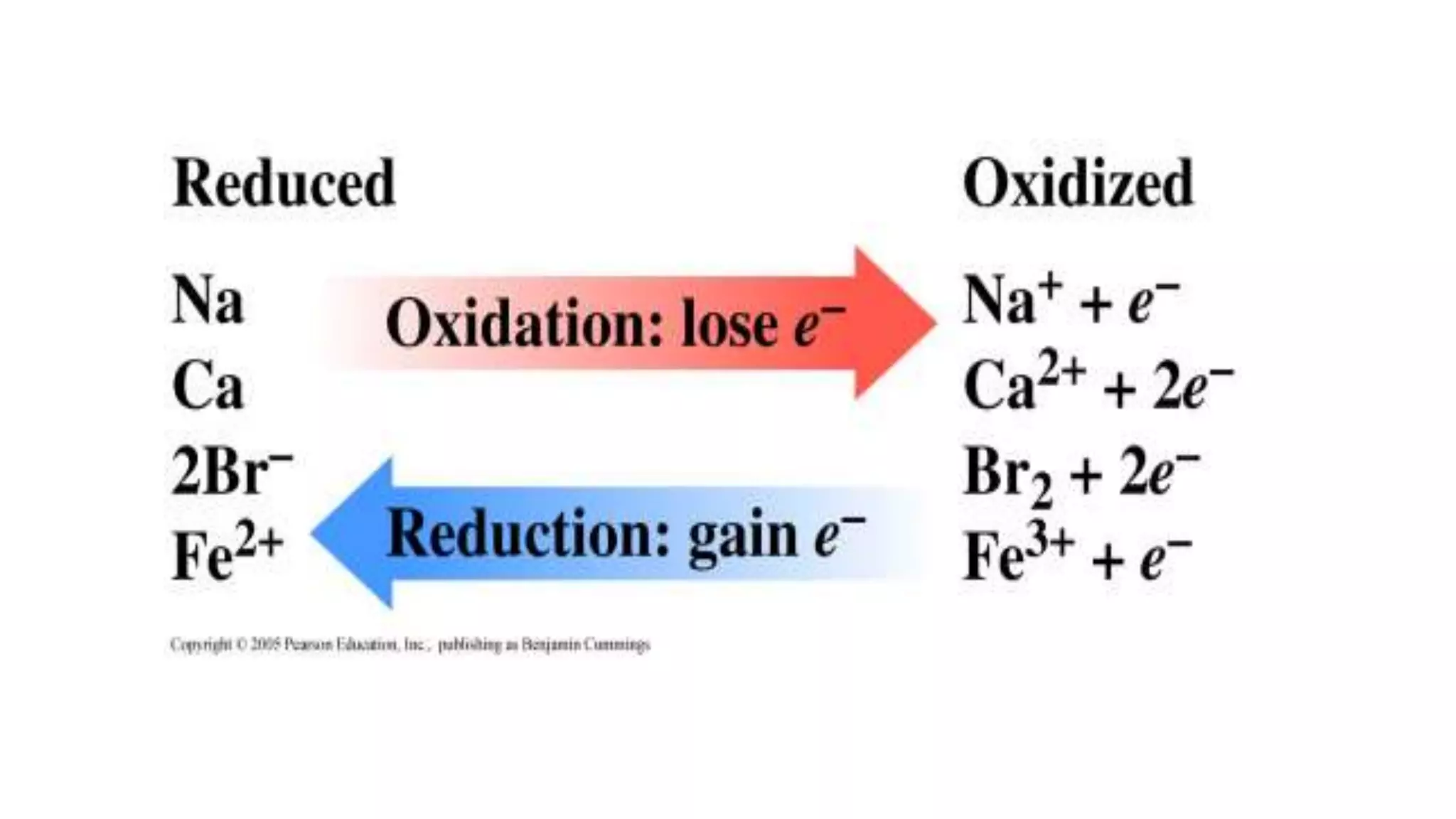
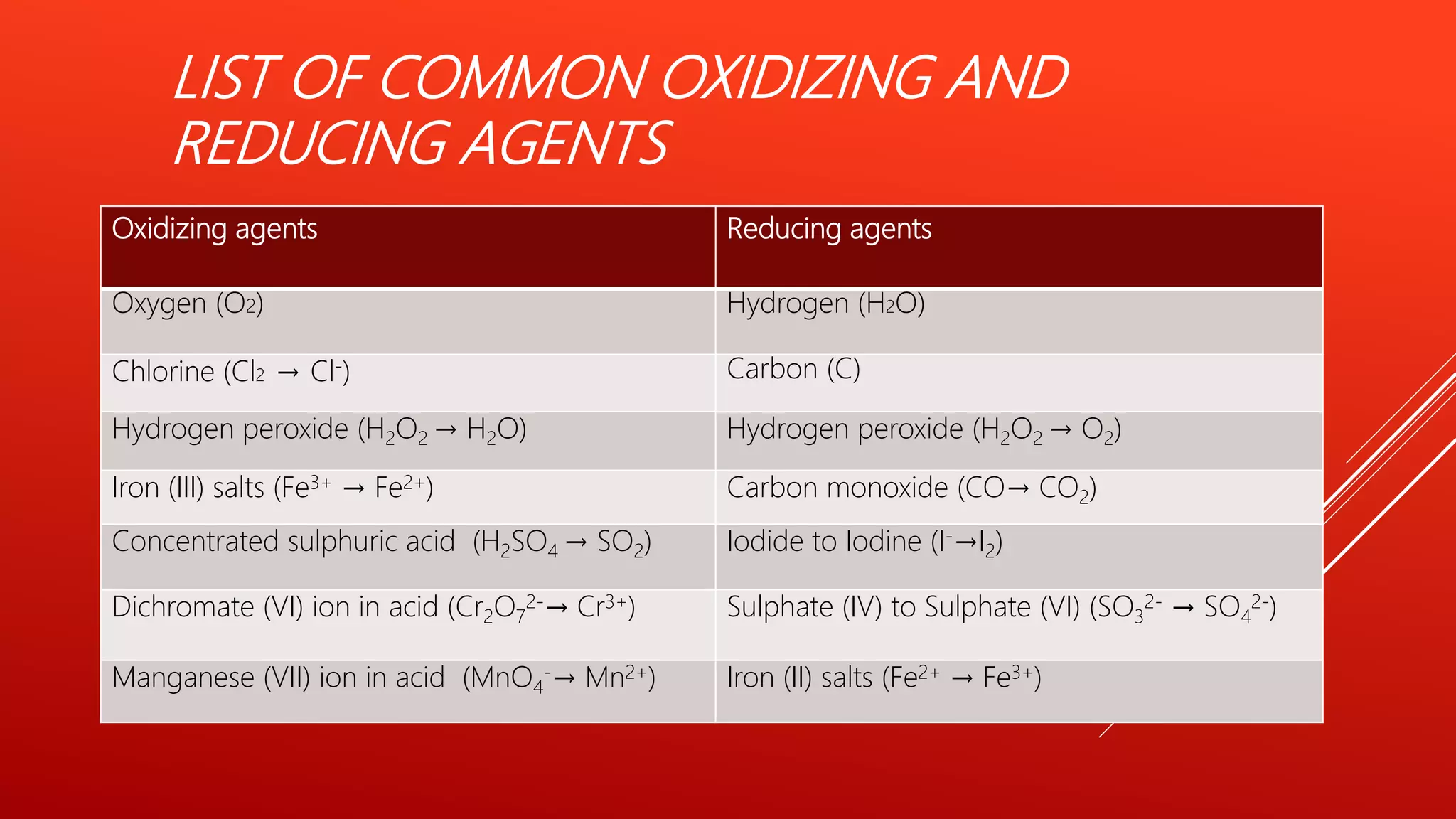
The document explains oxidation-reduction reactions, detailing the definitions and examples of oxidation and reduction processes, including the role of oxidation numbers. It also provides methods for determining oxidation states of various elements and identifies common oxidizing and reducing agents. Additionally, it includes sample questions for practice on identifying oxidizing and reducing agents in chemical reactions.