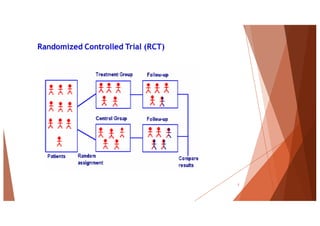
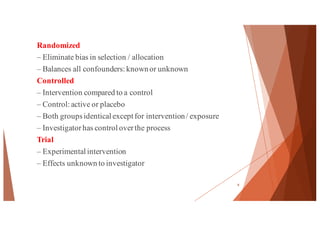
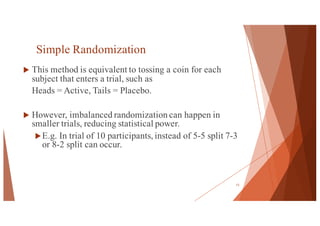
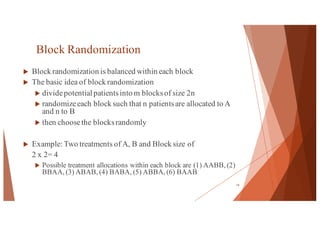
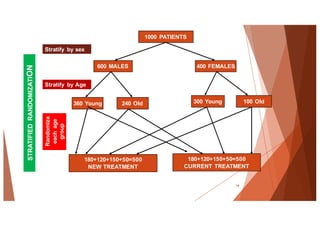
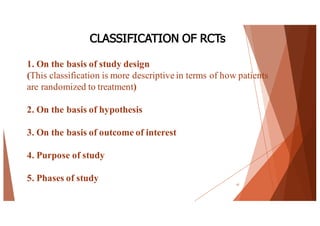
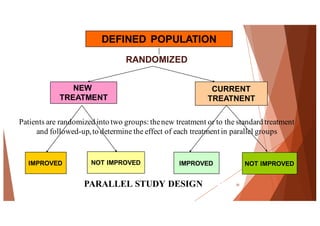
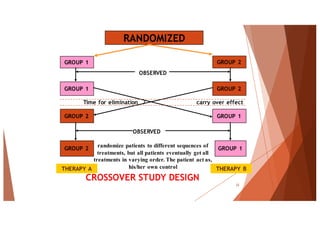
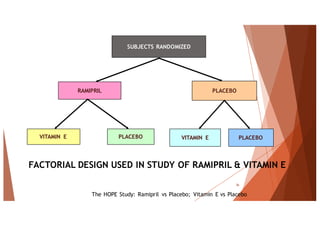
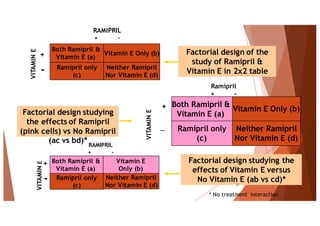
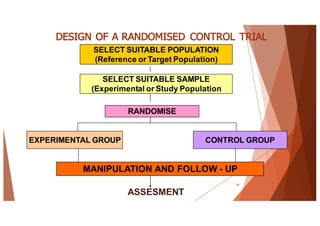
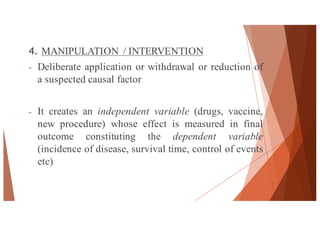
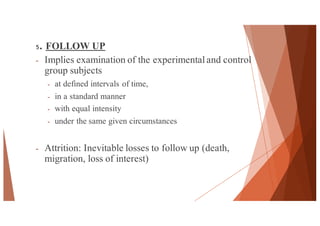
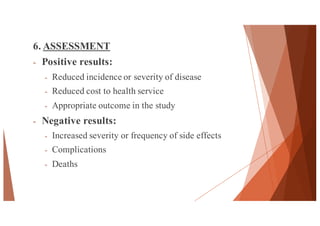
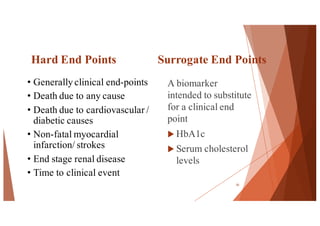
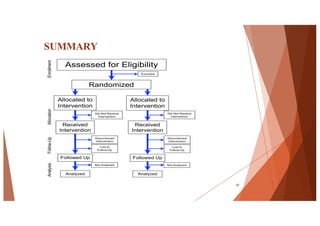
The document outlines the principles and methodologies of randomized controlled trials (RCTs), highlighting their importance in clinical research for evaluating the effectiveness of interventions. It covers the procedures for randomization, various study designs, ethical considerations, and the phases of clinical trials, along with advantages and disadvantages of RCTs. Key components such as allocation concealment, blinding, and endpoint measurements are also discussed, emphasizing their role in minimizing bias and ensuring valid results.