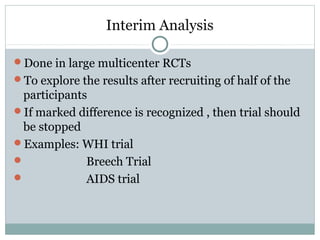
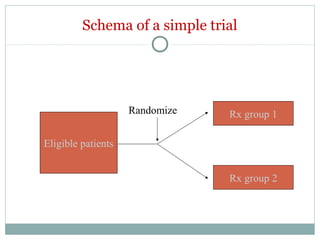
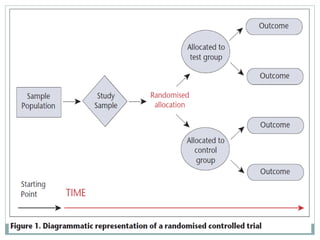
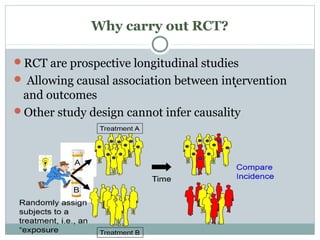
This document provides an overview of randomized controlled trials (RCTs). It defines RCTs as studies that compare two interventions by randomly assigning participants into groups. The key aspects covered include the importance of randomization for minimizing bias, common types of bias in RCTs, techniques for randomization, and ethical considerations. RCTs are considered the gold standard for inferring causality between an intervention and outcomes.






























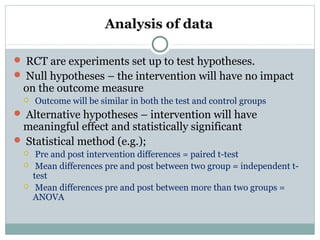
![Analysis of data
For dichotomous (binary) outcome data:
logistic regression (e.g., to predict sustained virological
response after receipt of peginterferon alfa-2a for hepatitis C)
and other methods can be used.
For continuous outcome data:
analysis of covariance (e.g., for changes in blood lipid levels after
receipt of atorvastatin after acute coronary syndrome[57]
) tests
the effects of predictor variables.
For time-to-event outcome data :
censored, survival analysis (e.g., Kaplan–Meier
estimators and Cox proportional hazards models for time
to coronary heart disease after receipt of hormone replacement
therapy in menopause[58]
) is appropriate.](https://image.slidesharecdn.com/rct-picsepsept15th2017-180218173600/85/Randomized-Controlled-Trials-57-320.jpg)