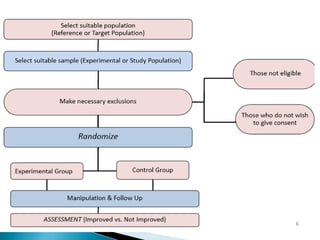
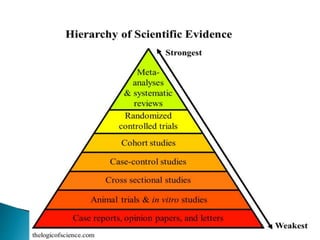
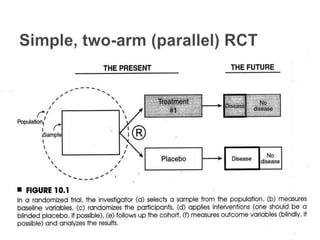
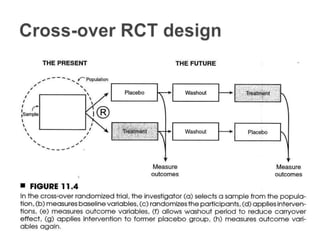
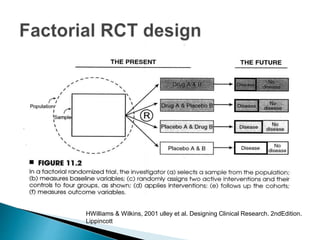
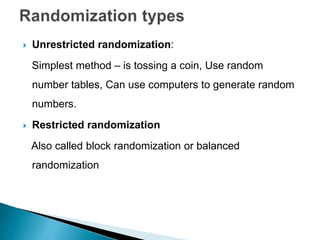
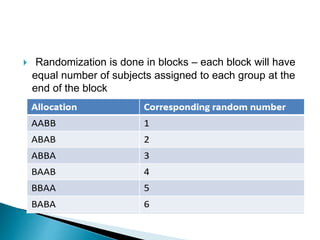
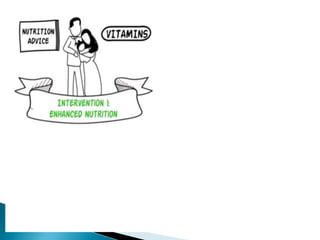
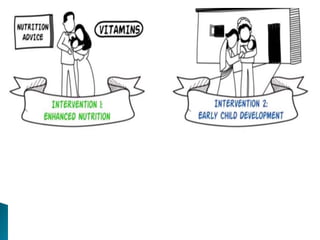
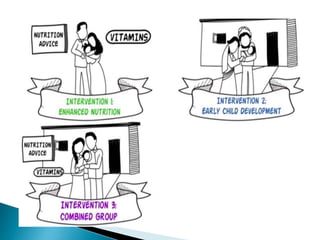
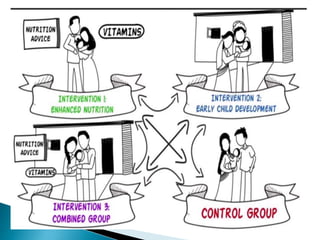
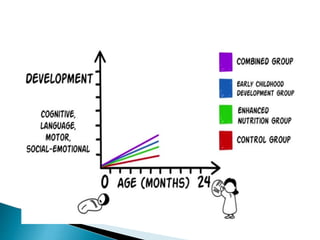
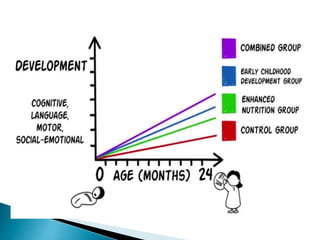
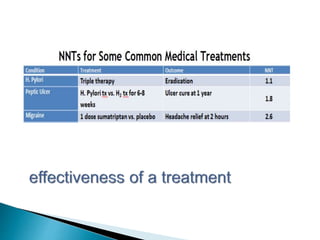
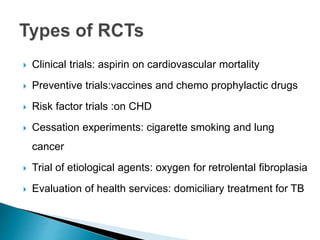
This document provides an overview of randomized controlled trials (RCTs), including their purpose and design. It discusses key aspects of RCTs such as randomization, blinding, and assessing outcomes. It provides examples of simple two-arm and cross-over RCT designs. The document also summarizes a specific cluster RCT that evaluated the effects of various child development and nutrition interventions on outcomes measured in children from birth to age 2 years.