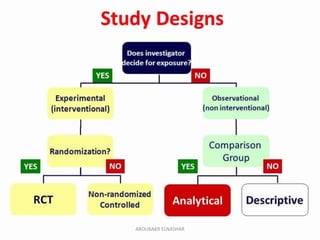
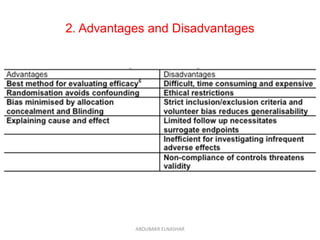
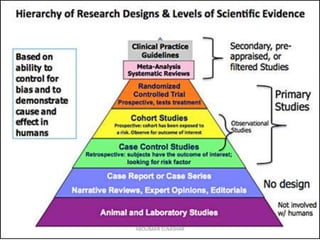
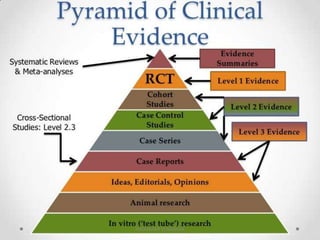
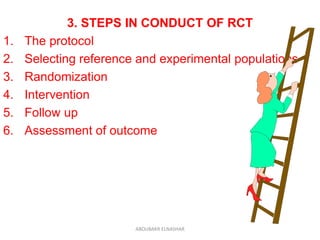
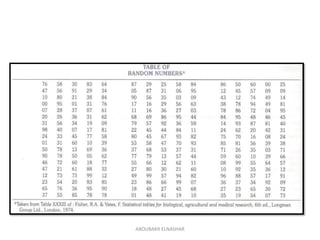
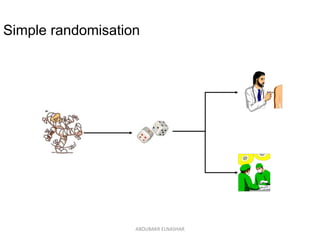
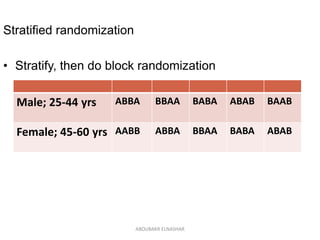
This document discusses randomized controlled trials (RCTs). It defines RCTs as epidemiological experiments that randomly allocate subjects to study and control groups to receive or not receive an experimental procedure or intervention. The document outlines the advantages of RCTs in evaluating treatments and determining causality. It describes the typical steps in conducting an RCT, including developing a protocol, randomizing subjects, administering interventions, following up, and assessing outcomes. It also discusses types of RCTs, ethical considerations, and ways to assess RCT quality.






















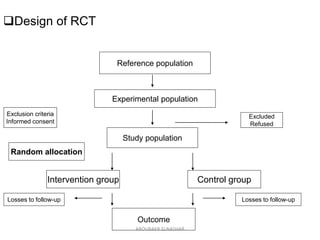
![Flowchart of 4 phases
(enrollment, intervention
allocation, follow-up, and
data analysis) of a
parallel randomized trial
of two groups, modified
from the CONSORT
(Consolidated Standards
of Reporting Trials) 2010
Statement[1]
ABOUBAKR ELNASHAR](https://image.slidesharecdn.com/rct2017-171019055630/85/Randomized-controlled-trials-Aboubakr-Elnashar-32-320.jpg)






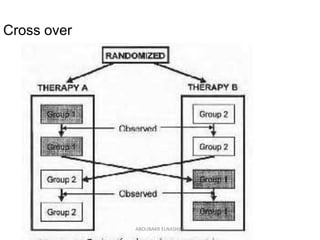

![An analysis of the 616 RCTs indexed in PubMed during December 2006 found that 78%
were parallel-group trials, 16% were crossover, 2% were split-body, 2% were cluster,
and 2% were factorial.[32]
ABOUBAKR ELNASHAR](https://image.slidesharecdn.com/rct2017-171019055630/85/Randomized-controlled-trials-Aboubakr-Elnashar-41-320.jpg)




















