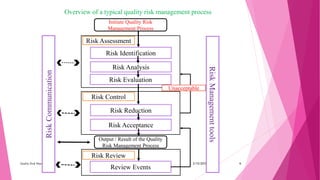
Mr. Gite Navnath Khandu presented on quality risk management. The presentation covered:
1) The principles of quality risk management including systematically assessing, controlling, communicating, and reviewing risks to drug quality across the product lifecycle.
2) The quality risk management process which includes steps like risk identification, analysis, evaluation, control, and review.
3) Integration of quality risk management into industry and regulatory operations like development, manufacturing, and inspections.