Embed presentation
Download to read offline





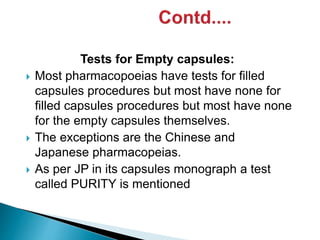


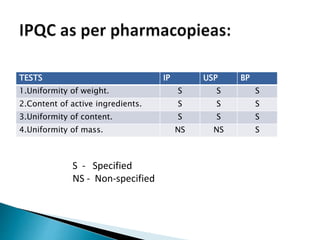
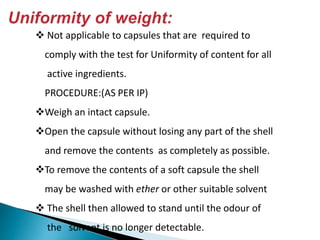

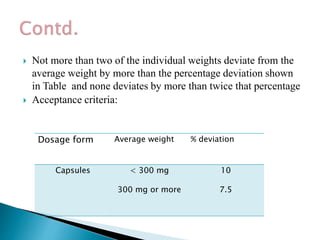


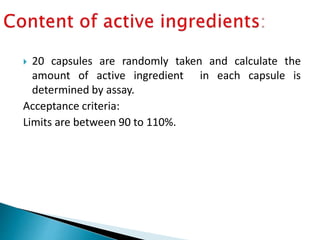


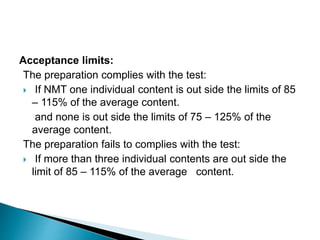
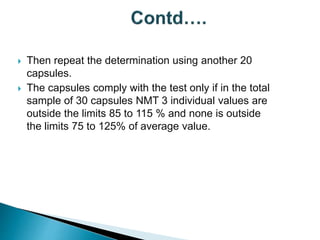
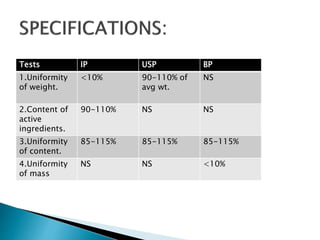


Capsules come in both hard and soft gelatin shells that enclose solid or liquid medications. Quality control tests are conducted on empty capsules and finished capsules to ensure uniformity of weight, content of active ingredients, and dissolution. Key tests include uniformity of weight, content of active ingredients, and uniformity of content. Acceptance criteria vary slightly between pharmacopeias but generally require less than 10% deviation from the average weight and 90-110% of the average active content. In-process quality checks are also important to monitor production and identify defects.






















