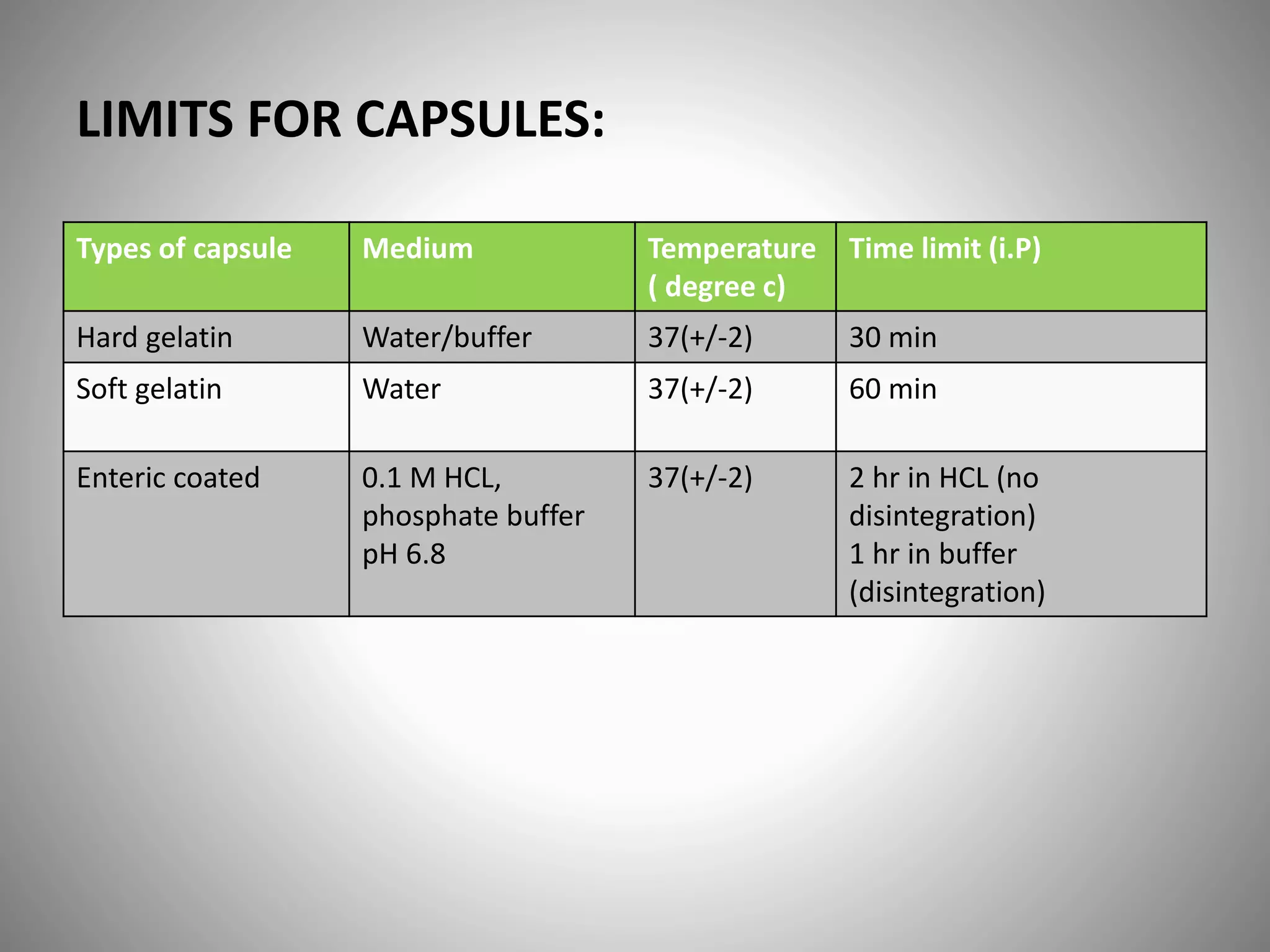
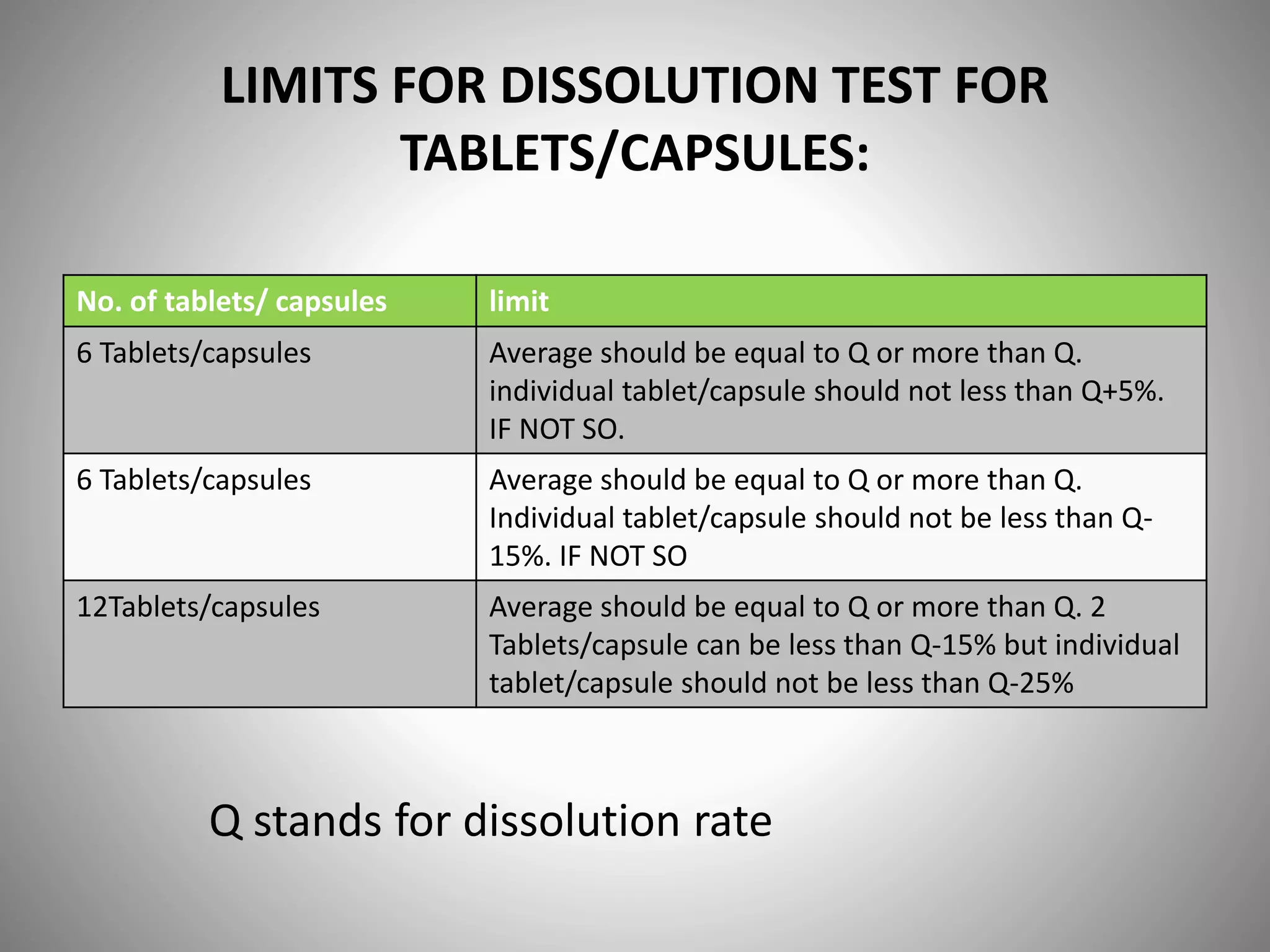
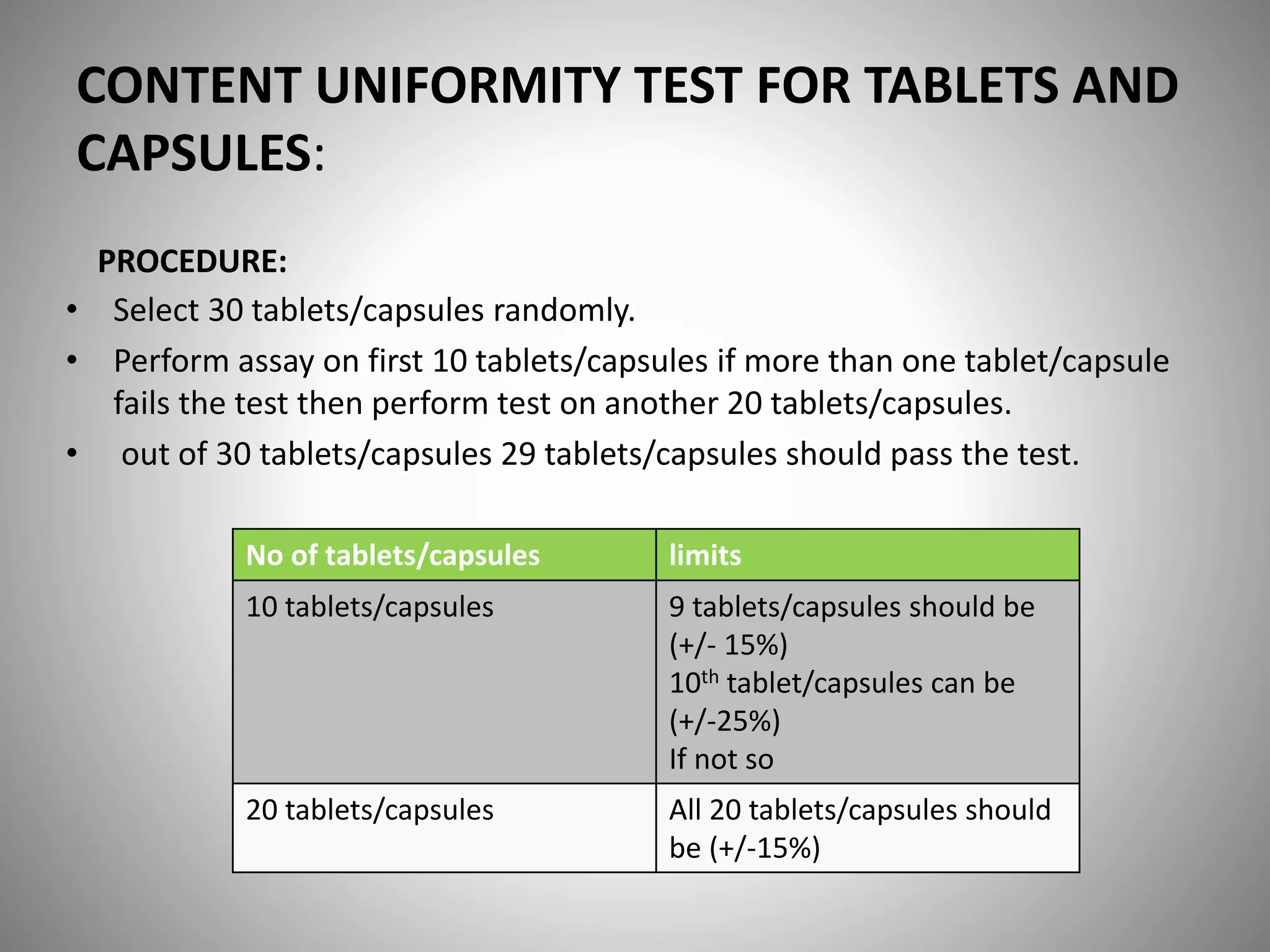
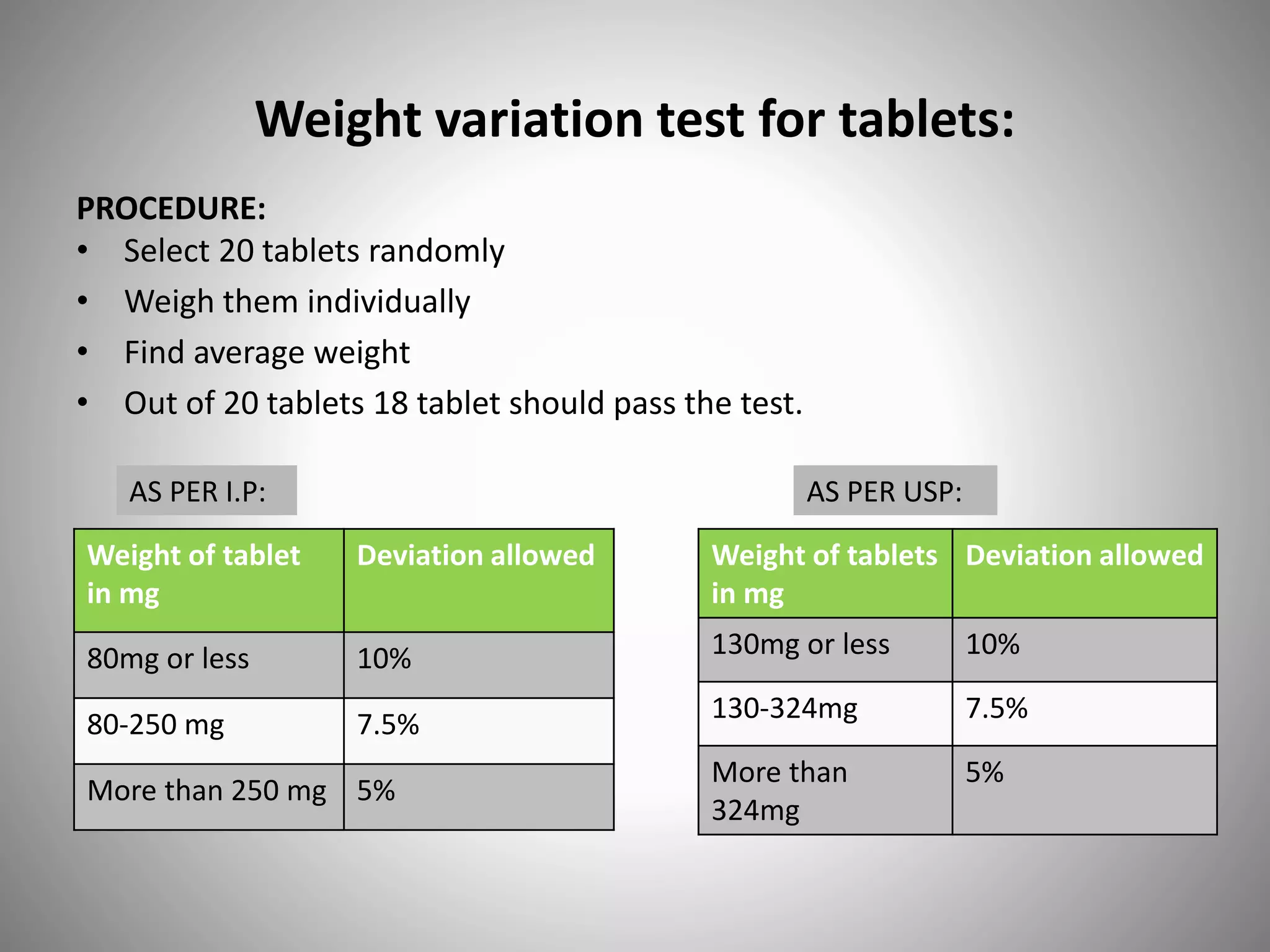
This document provides definitions and evaluation methods for solid oral dosage forms like tablets and capsules. It defines tablets as containing an active ingredient with or without excipients prepared by molding or compression, and capsules as containing the active ingredient filled into a cap and body. It describes methods like disintegration testing using baskets, dissolution testing using paddle or basket apparatuses, content uniformity testing, weight variation testing, friability testing, and moisture permeation testing to evaluate important properties of tablets and capsules.