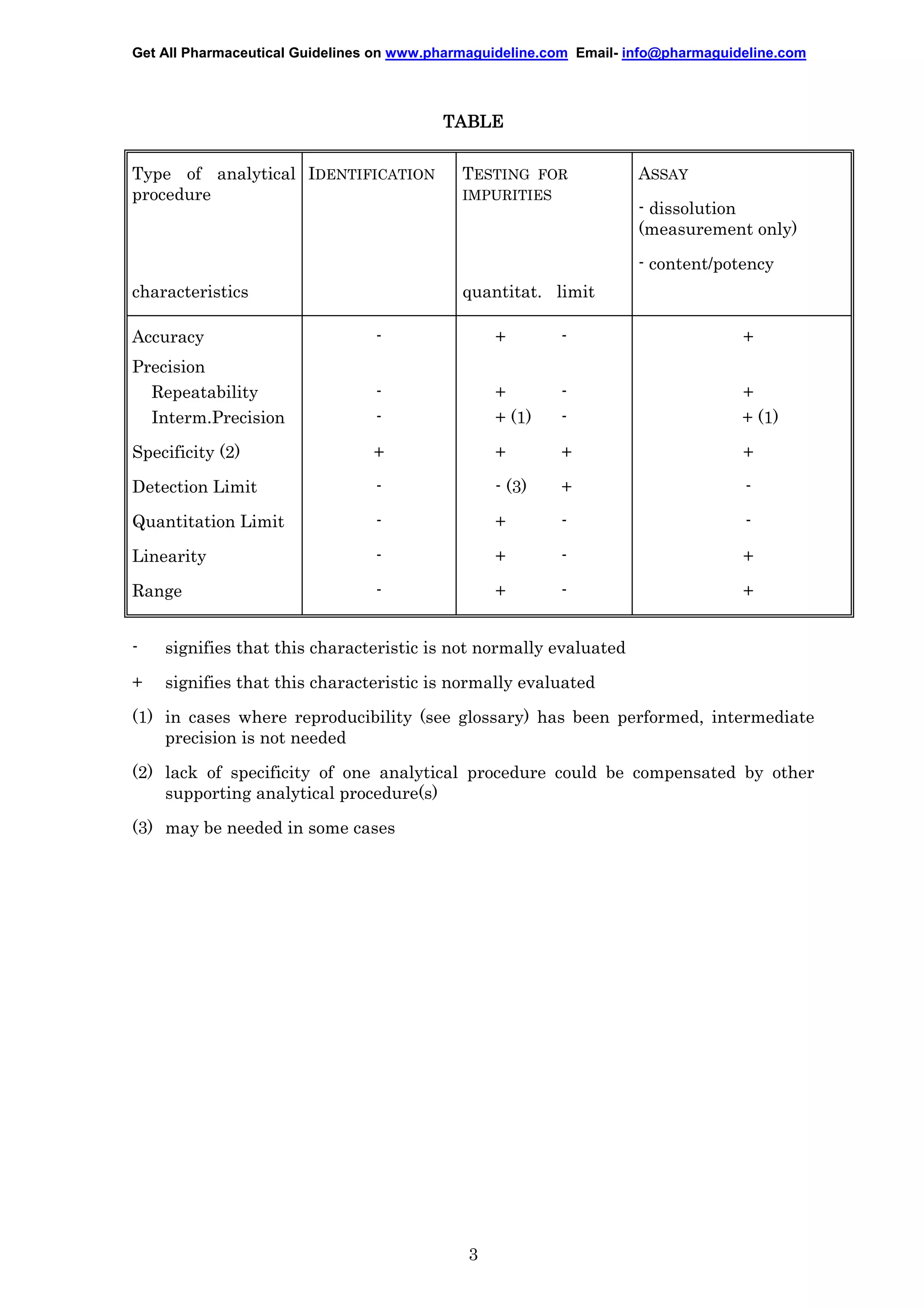
This document provides guidelines for validating analytical procedures used in pharmaceutical registration applications. It discusses four common types of analytical procedures: identification tests, impurity tests, limit tests for impurities, and quantitative assays. The guidelines describe key validation characteristics such as accuracy, precision, specificity, detection limit, quantitation limit, linearity, range, and robustness. It provides a table summarizing which characteristics are most important to evaluate for each type of analytical procedure. The document also includes definitions for validation terminology and methodology recommendations for demonstrating various validation characteristics.