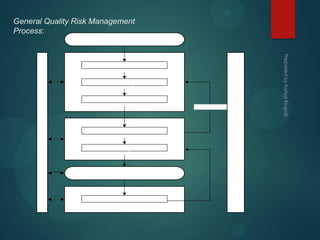
The document discusses the International Conference on Harmonization (ICH), which aims to harmonize technical requirements for pharmaceutical registration across regions to ensure safety and efficacy. ICH involves regulators and industry from the EU, Japan, and US. The objectives of ICH include increasing international harmonization, developing pharmaceuticals efficiently, promoting public health, and minimizing animal testing. ICH guidelines cover quality, efficacy, safety, and multidisciplinary topics.