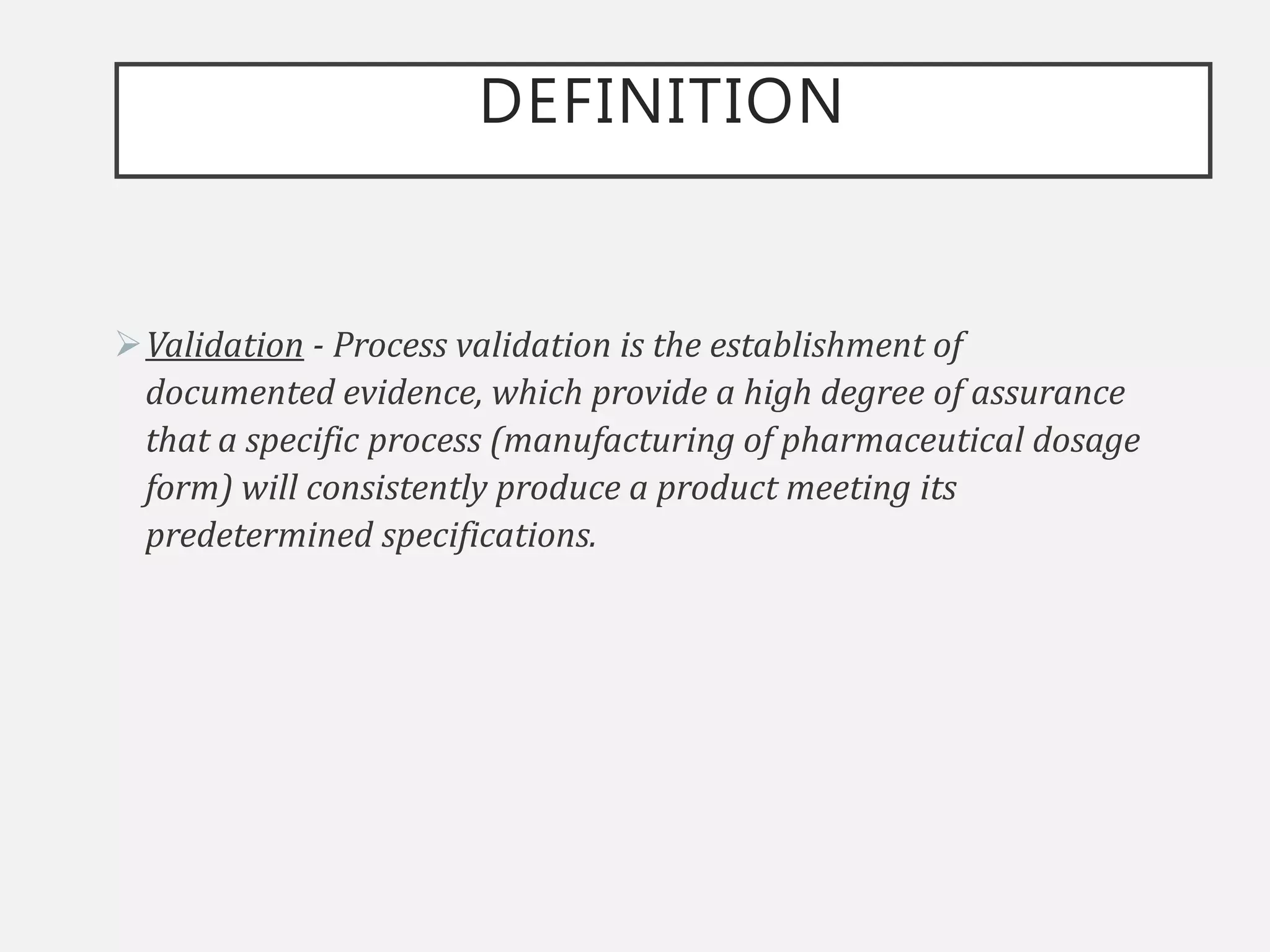
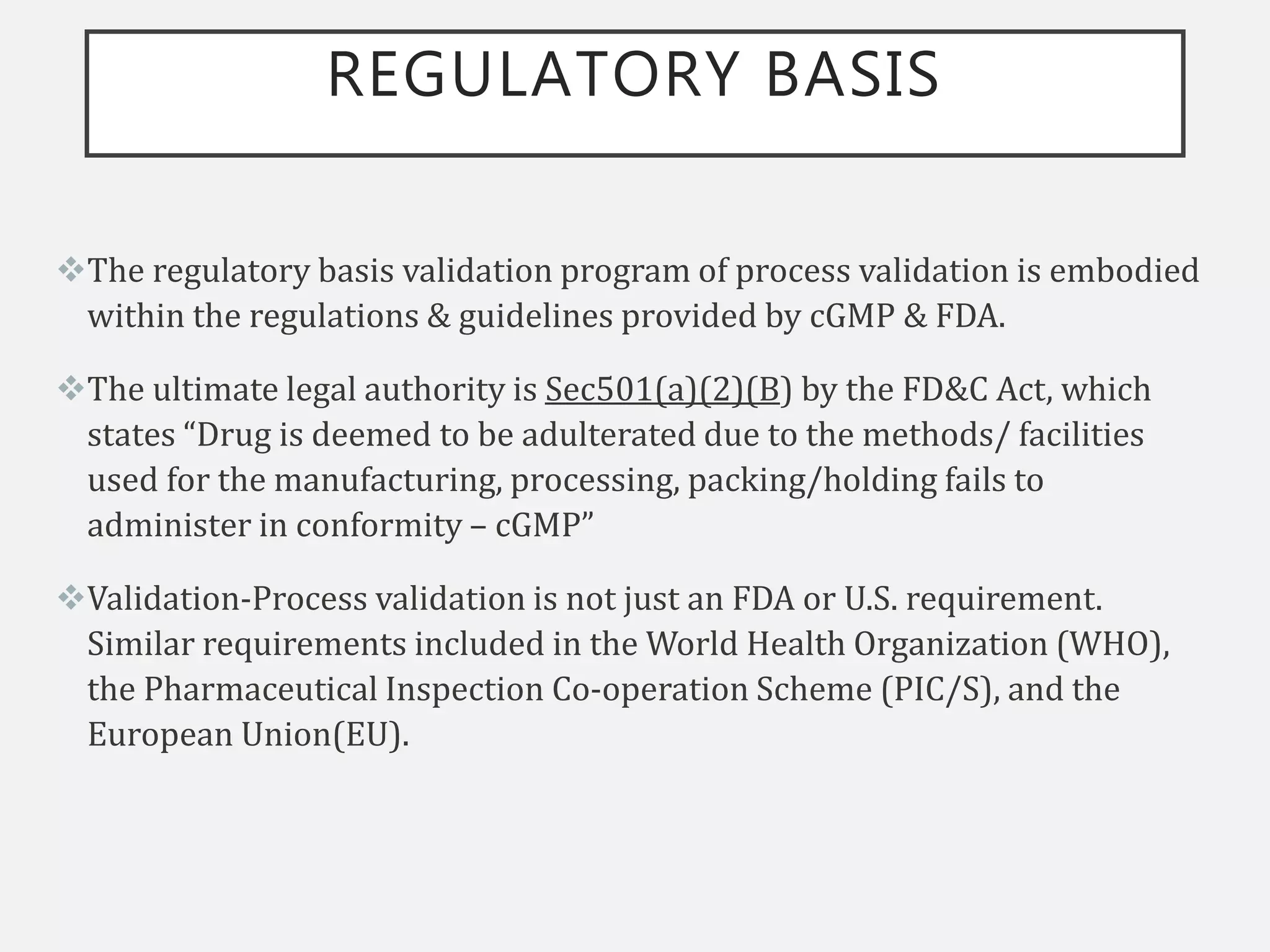
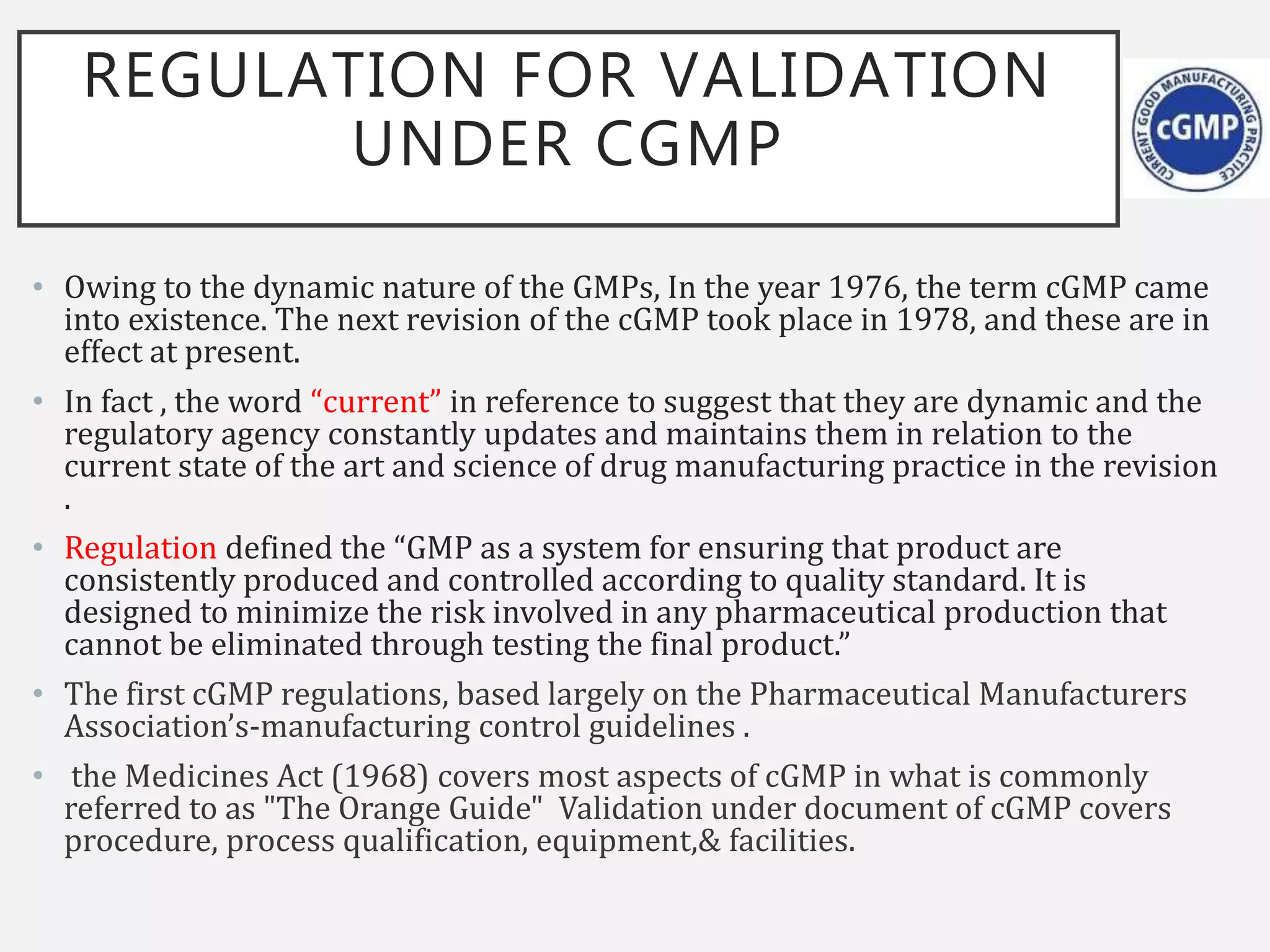
This document provides an overview of government regulations for pharmaceutical validation from various regulatory bodies such as the US FDA, cGMP, WHO, EU, and PIC/S. It defines validation, discusses the importance of validation, and outlines the historical background that led to regulation. The key points are that validation became mandatory under cGMP regulations to ensure quality and minimize risk, and all major regulatory bodies now require validation studies to be conducted according to predefined protocols and provide documented evidence that processes will consistently produce quality products meeting specifications.