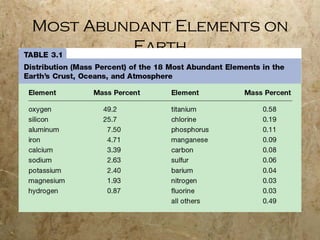
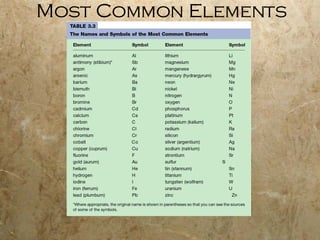
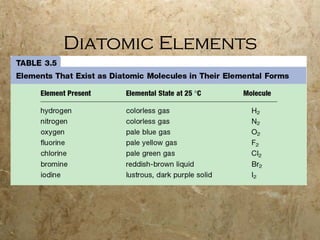
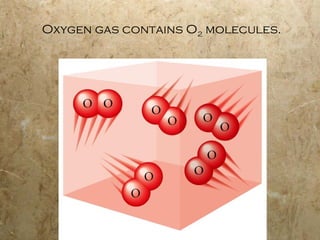
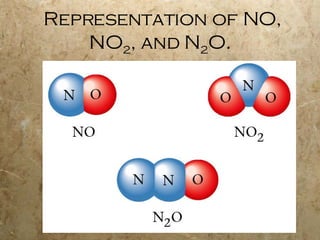
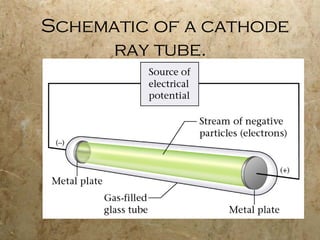
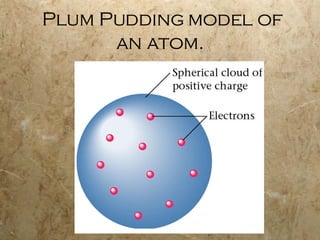
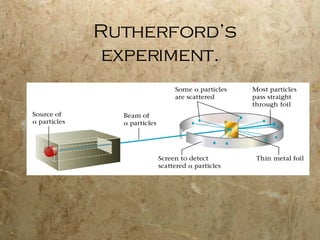
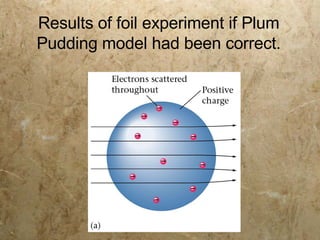
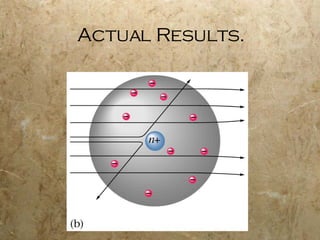
Atoms are the smallest particles of elements that retain their chemical properties. Atoms consist of protons, neutrons, and electrons. John Dalton proposed his atomic theory in 1808, stating that all matter is made of indivisible atoms, atoms of the same element are identical, and atoms combine in simple whole number ratios. In the early 1900s, experiments showed that atoms have a small, dense nucleus surrounded by electrons, and electrons can exist in certain energy levels or orbits around the nucleus.