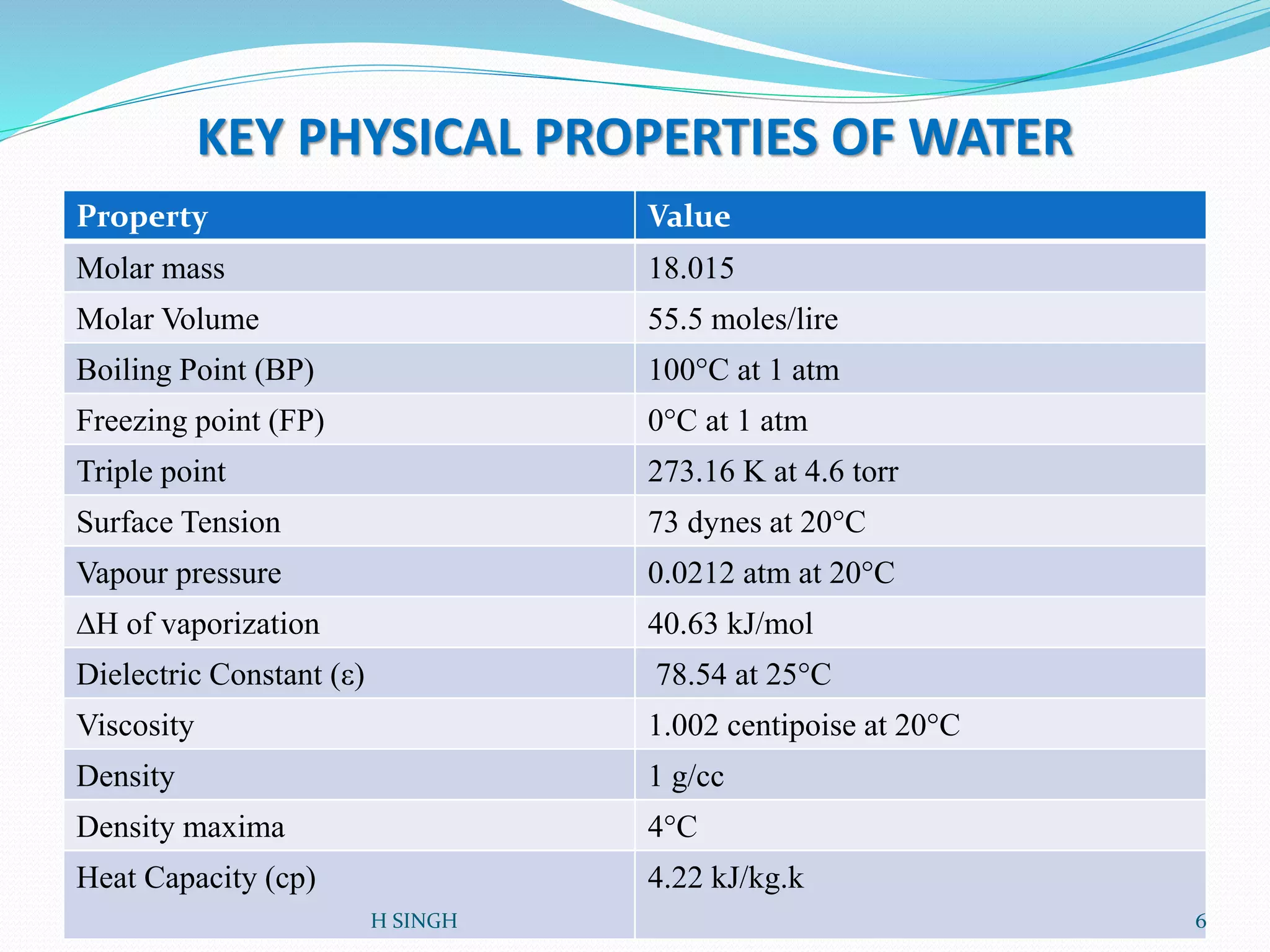
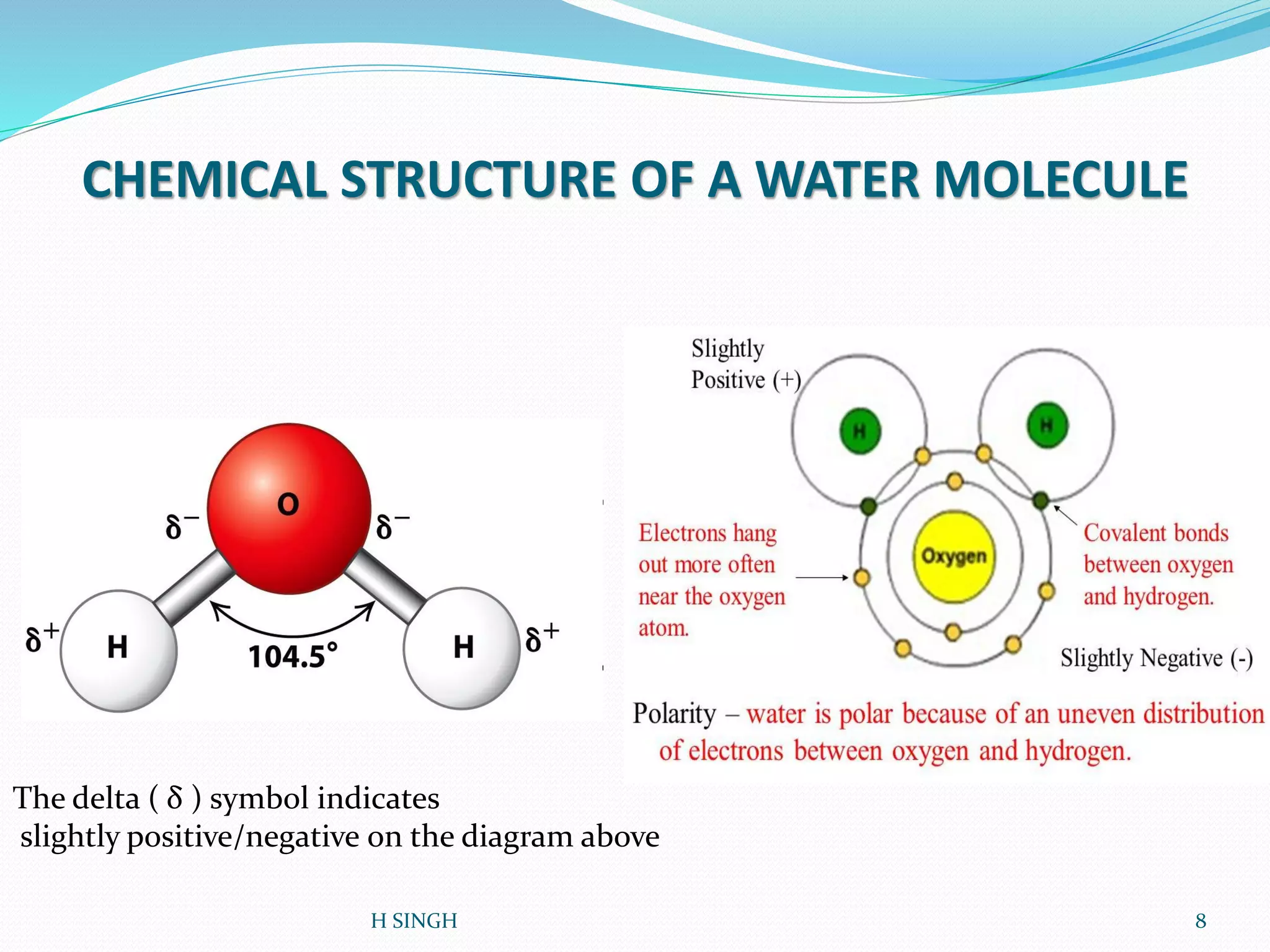
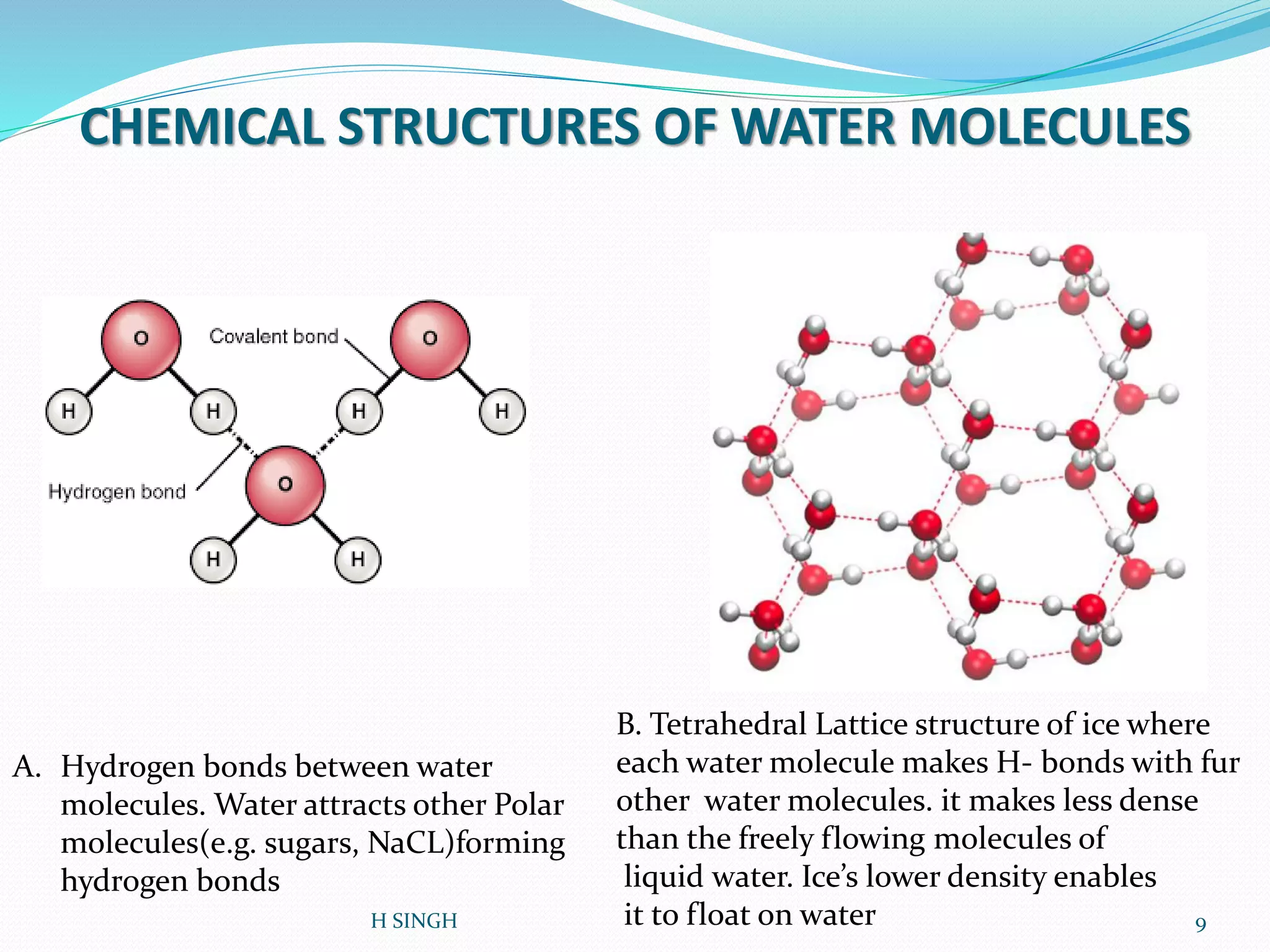
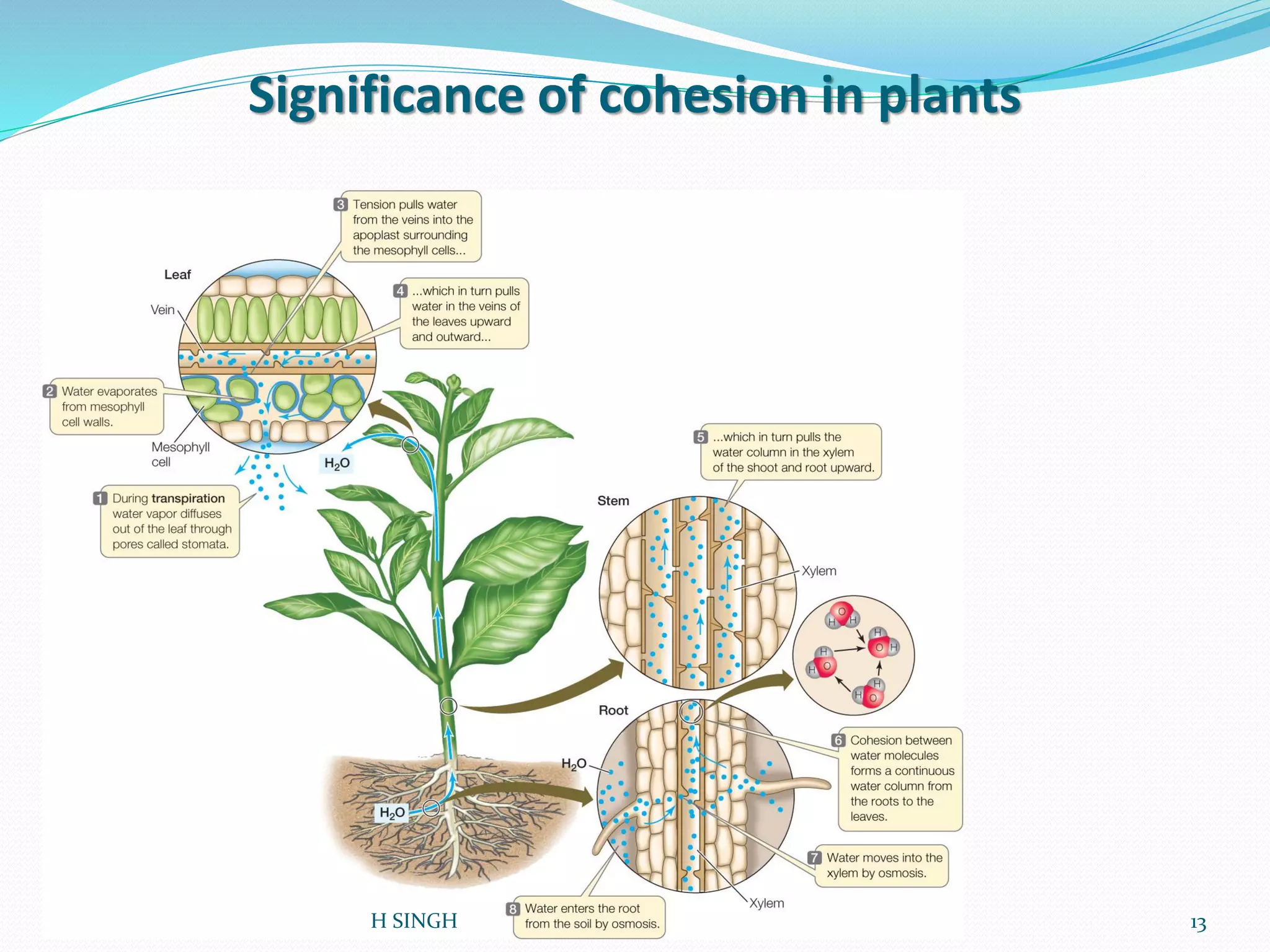
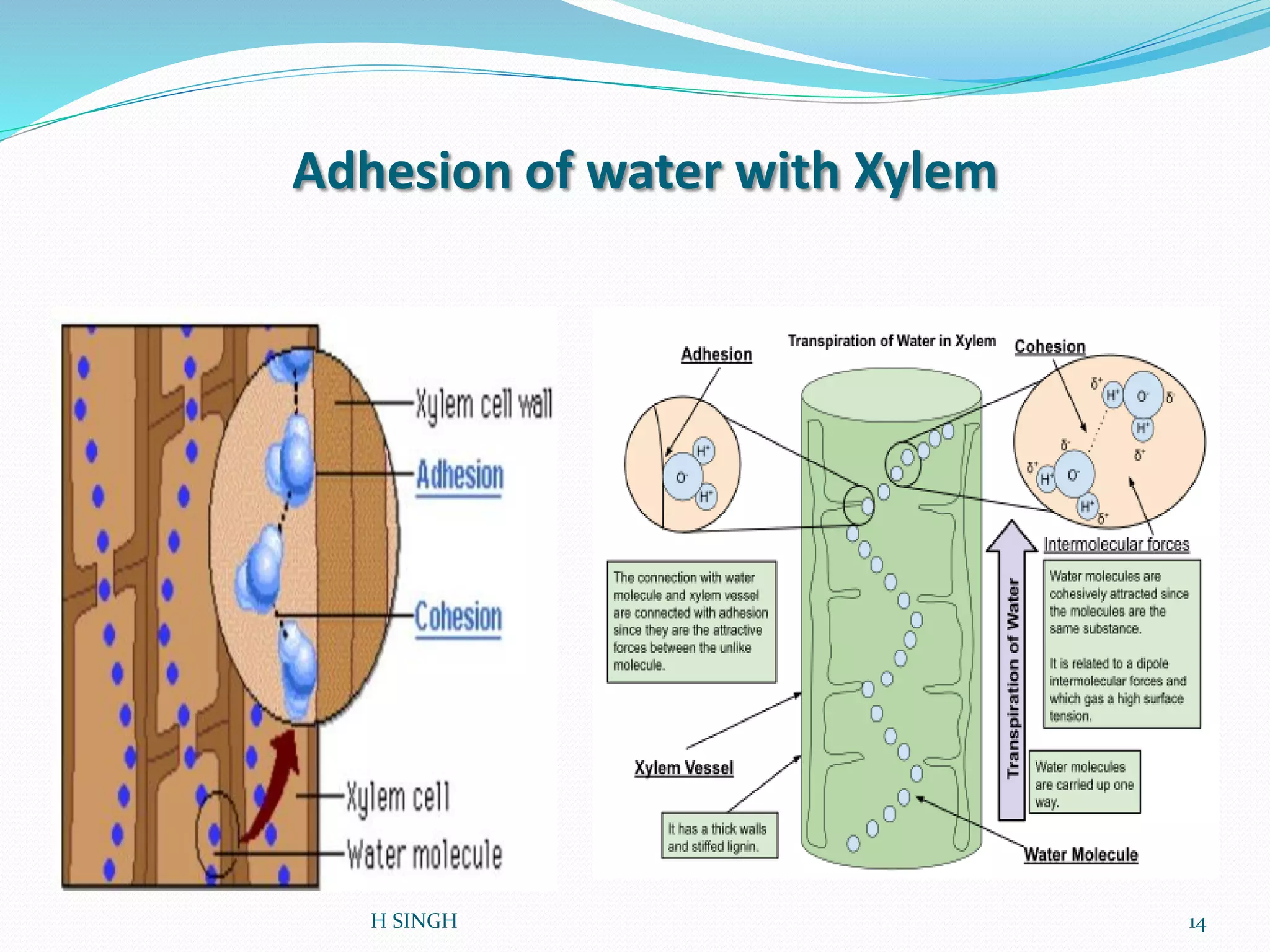
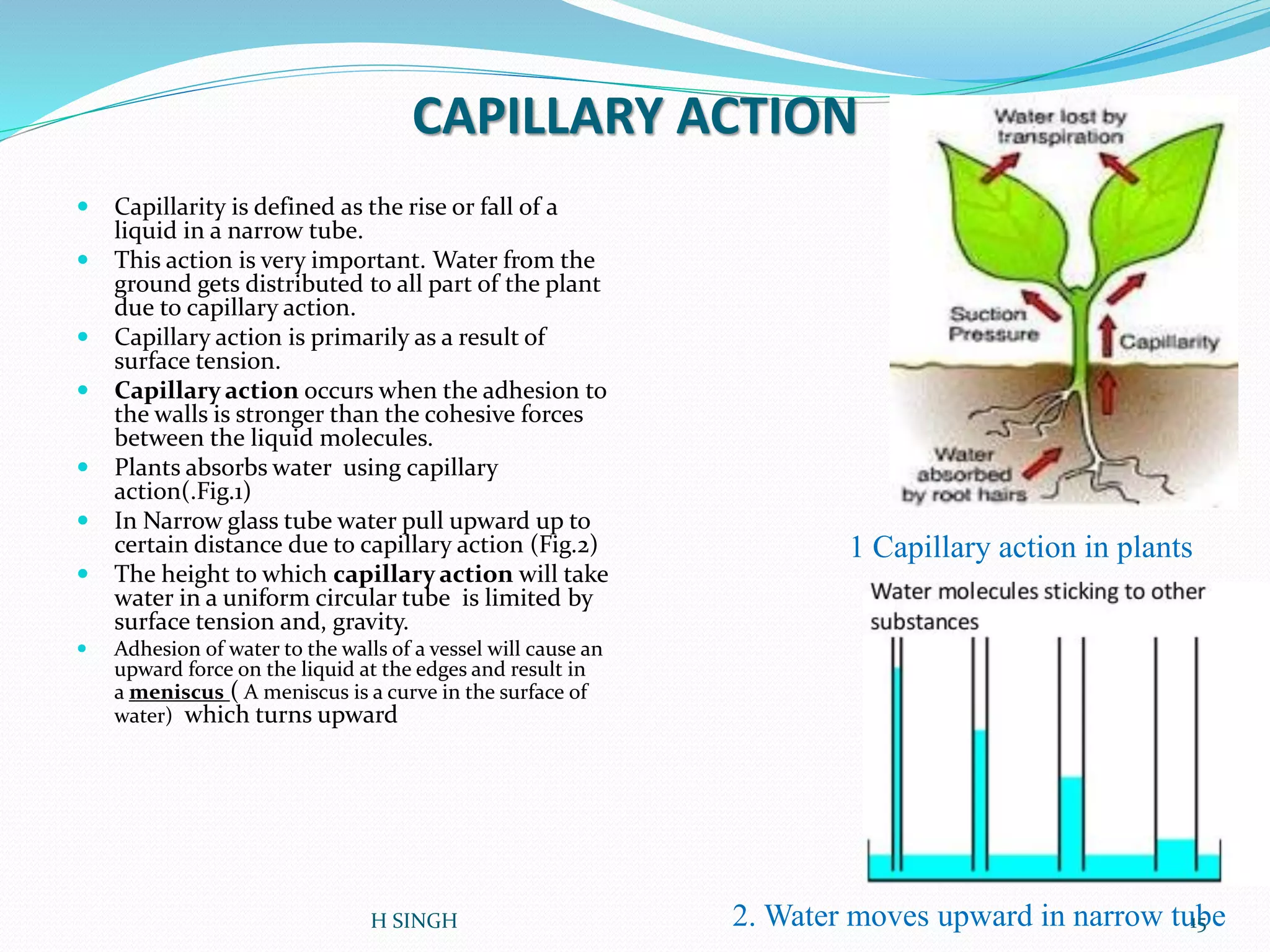
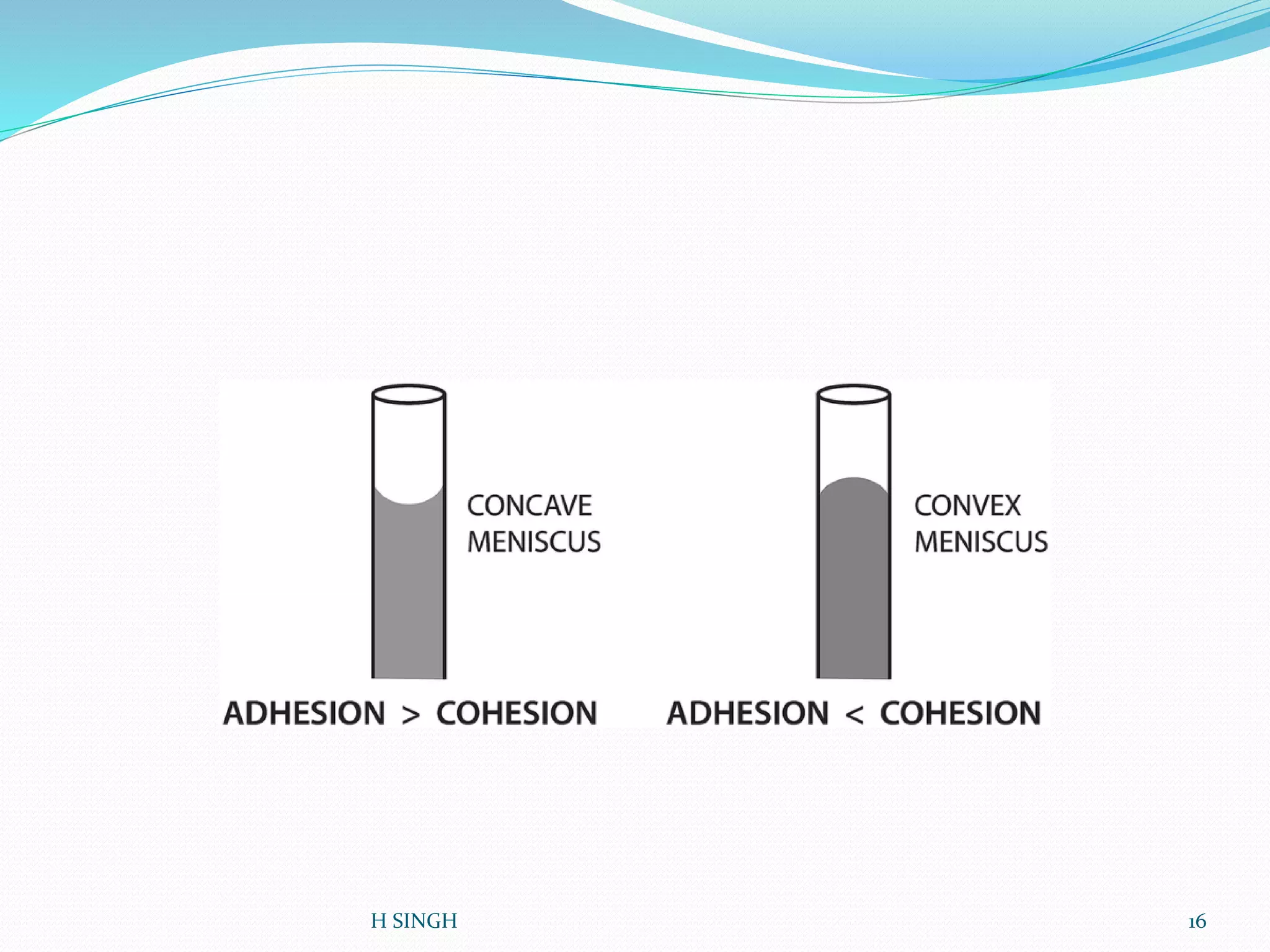
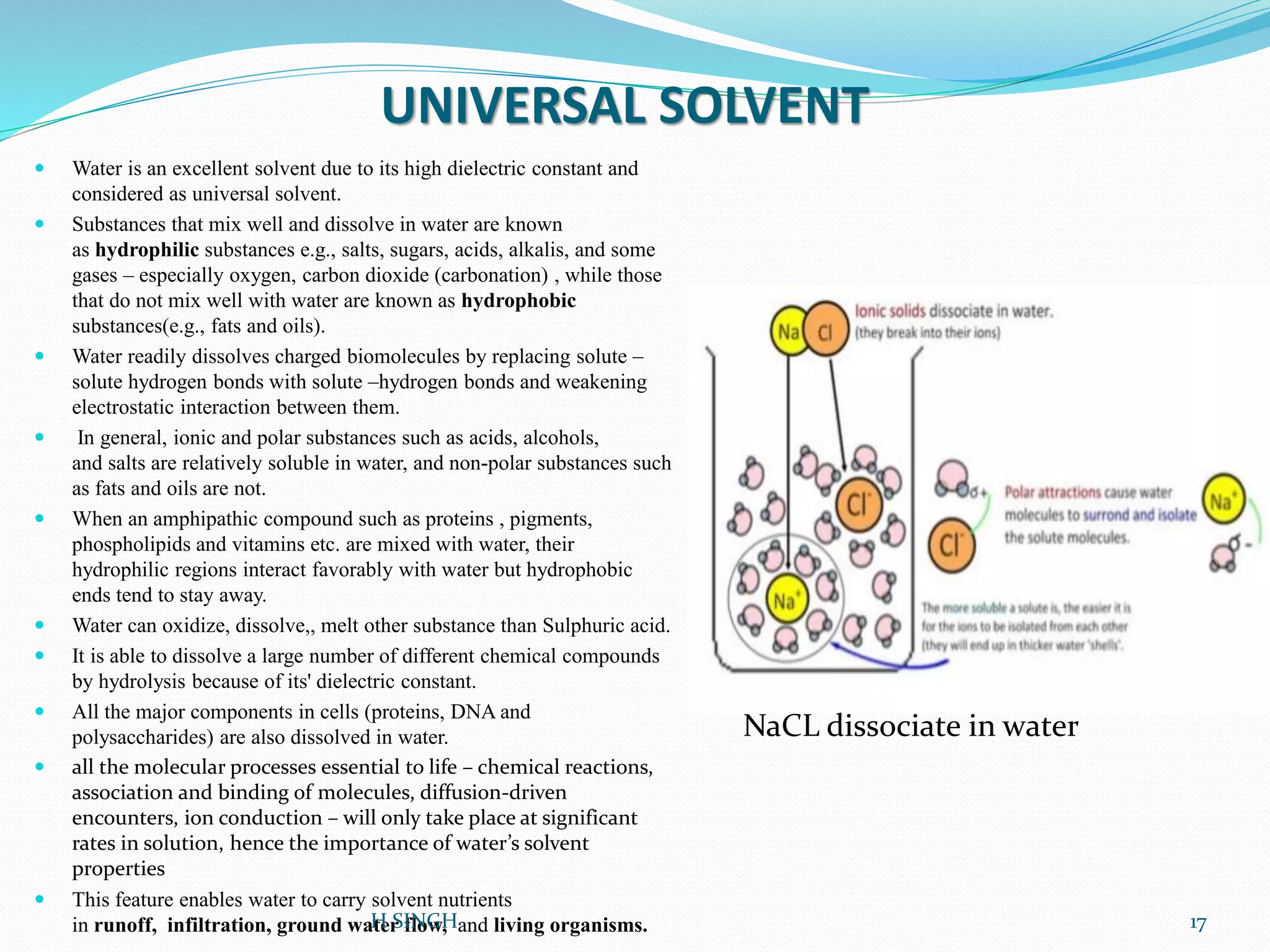
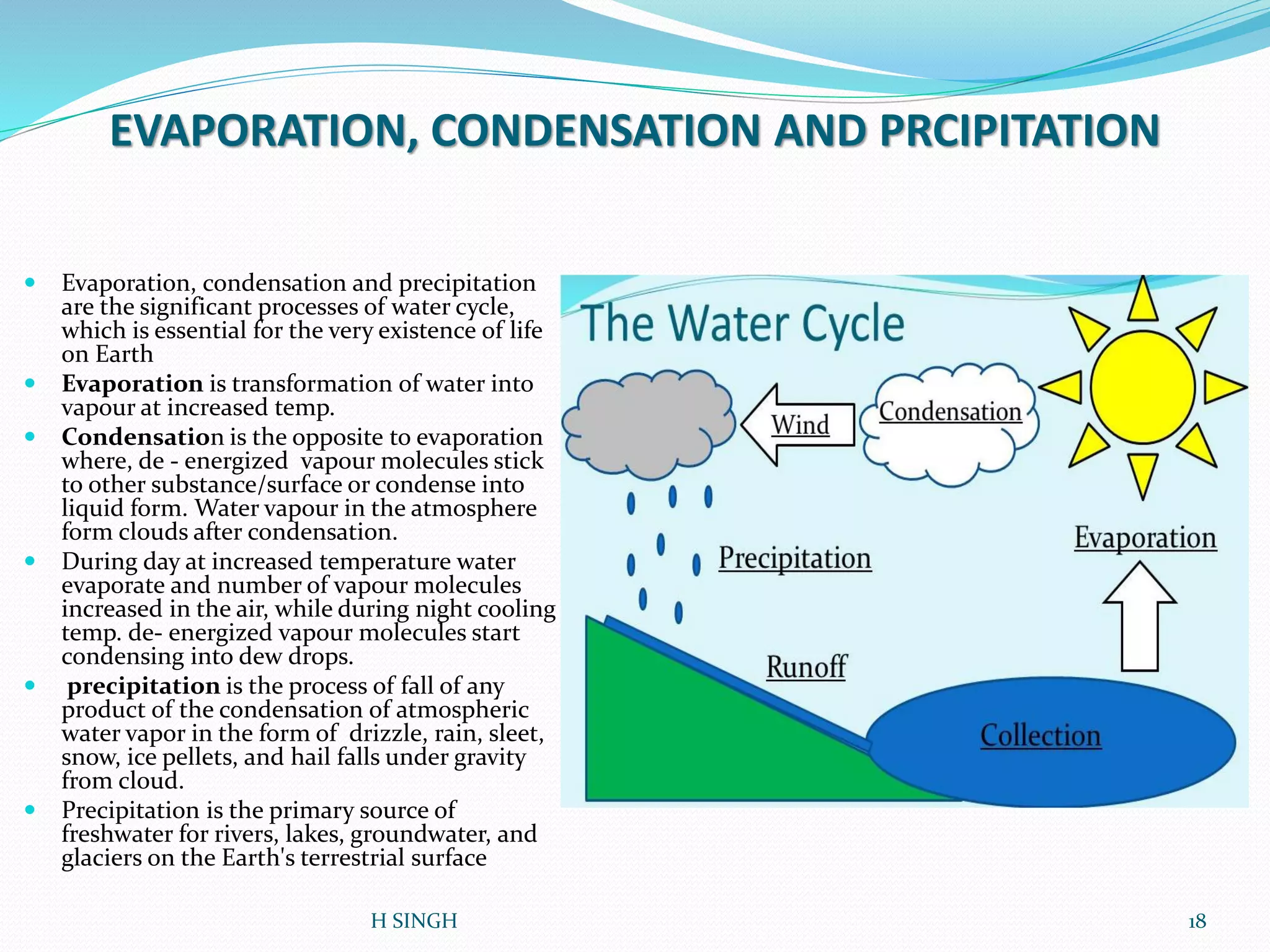
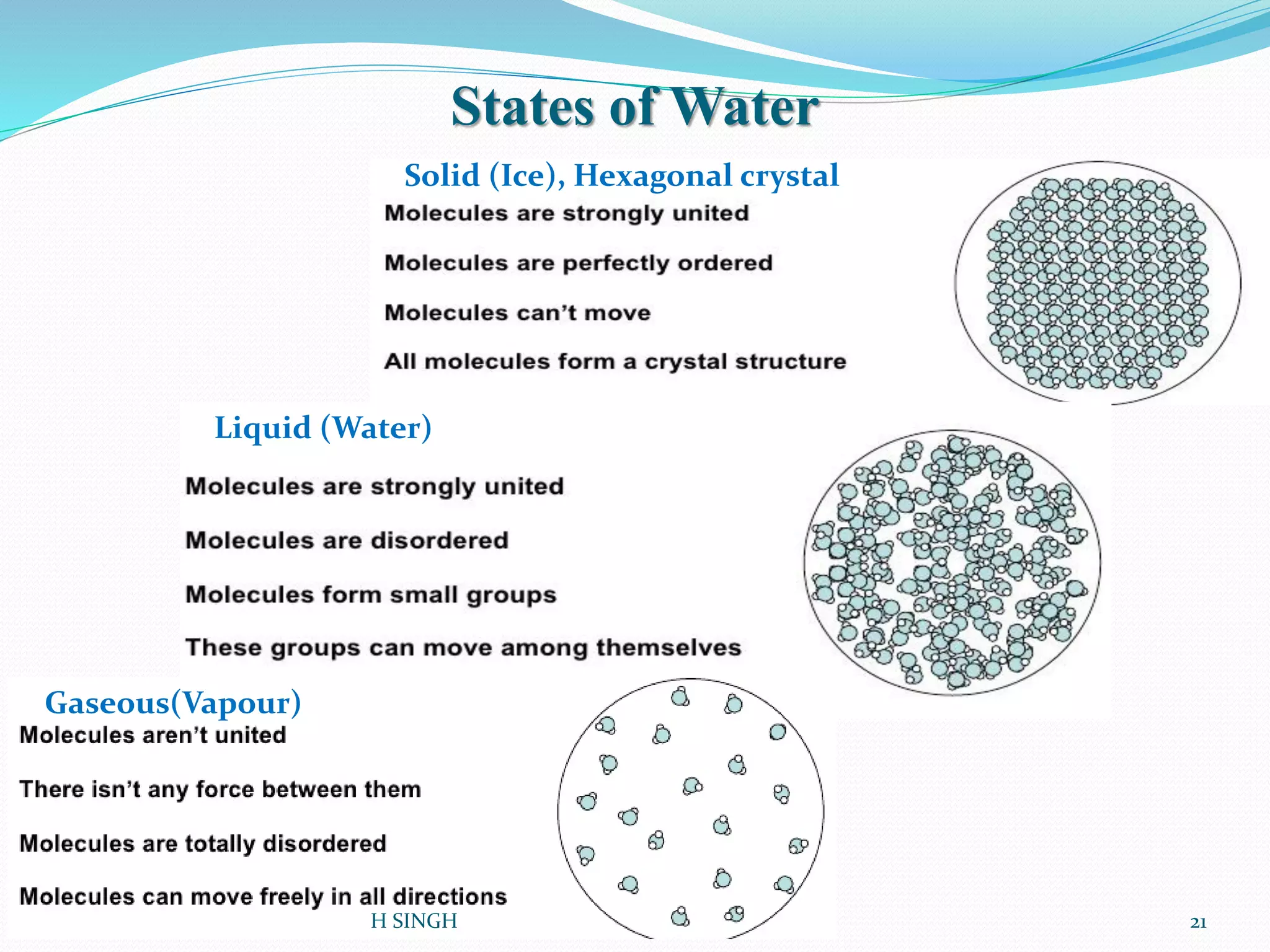
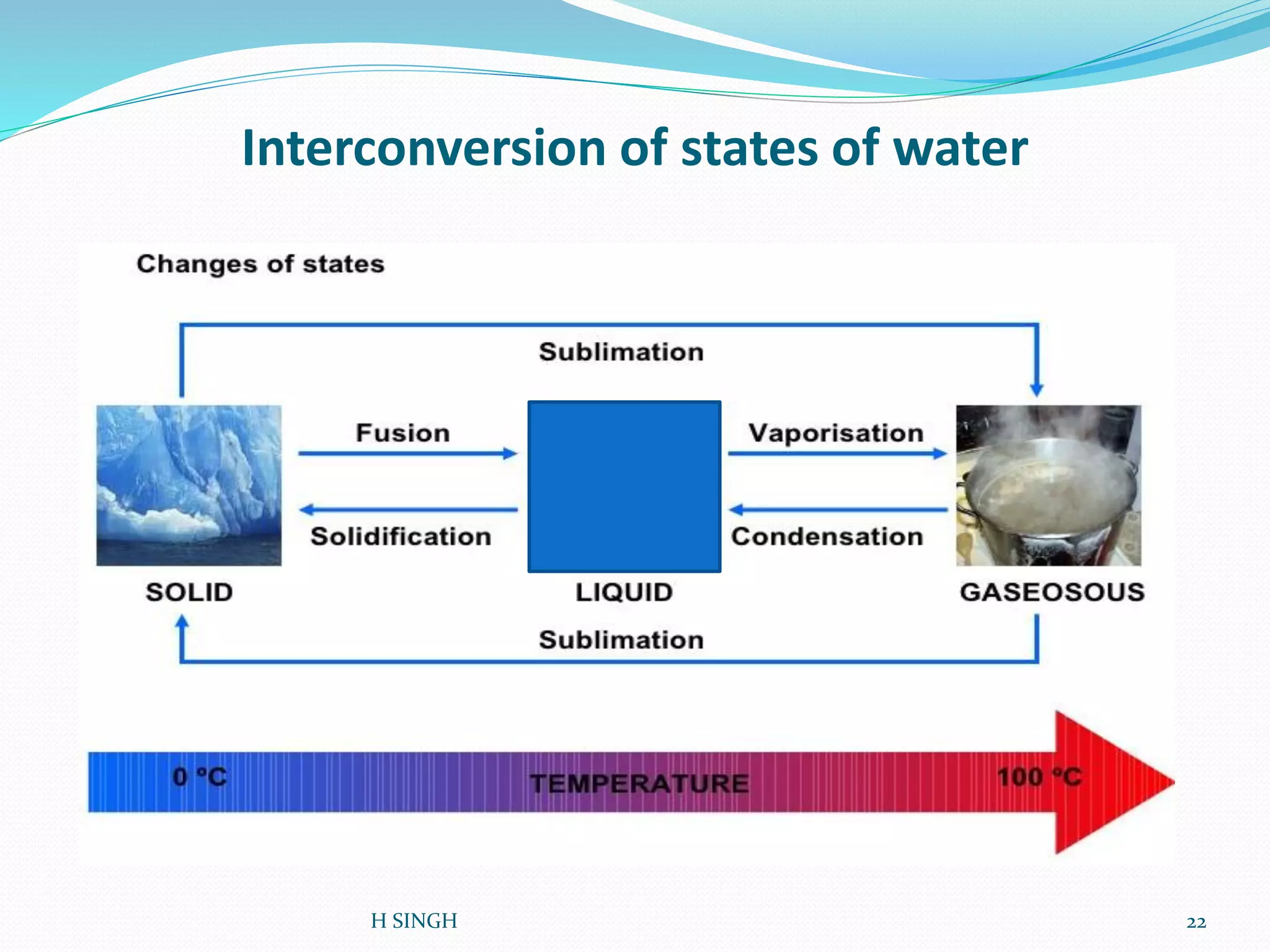
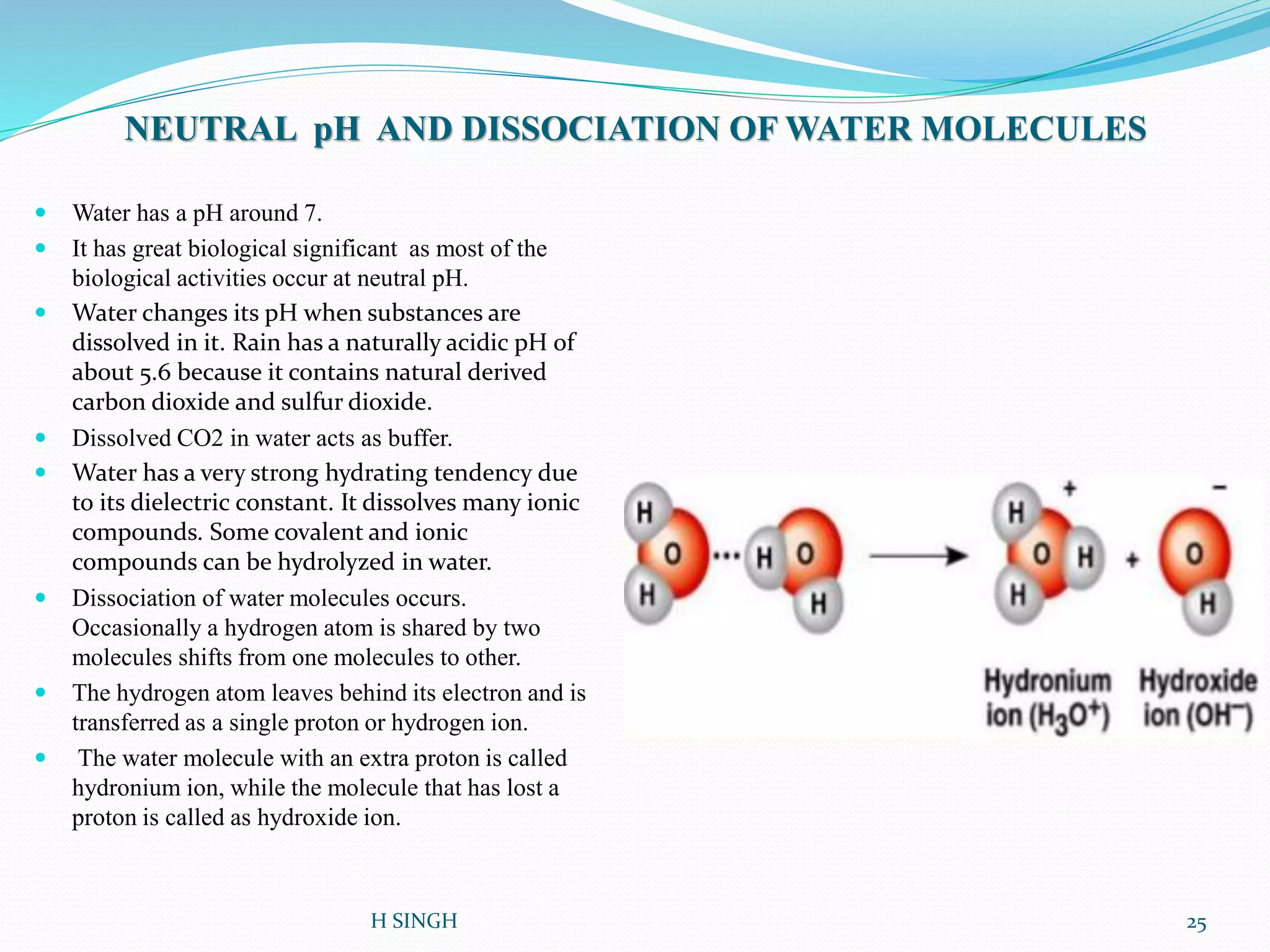
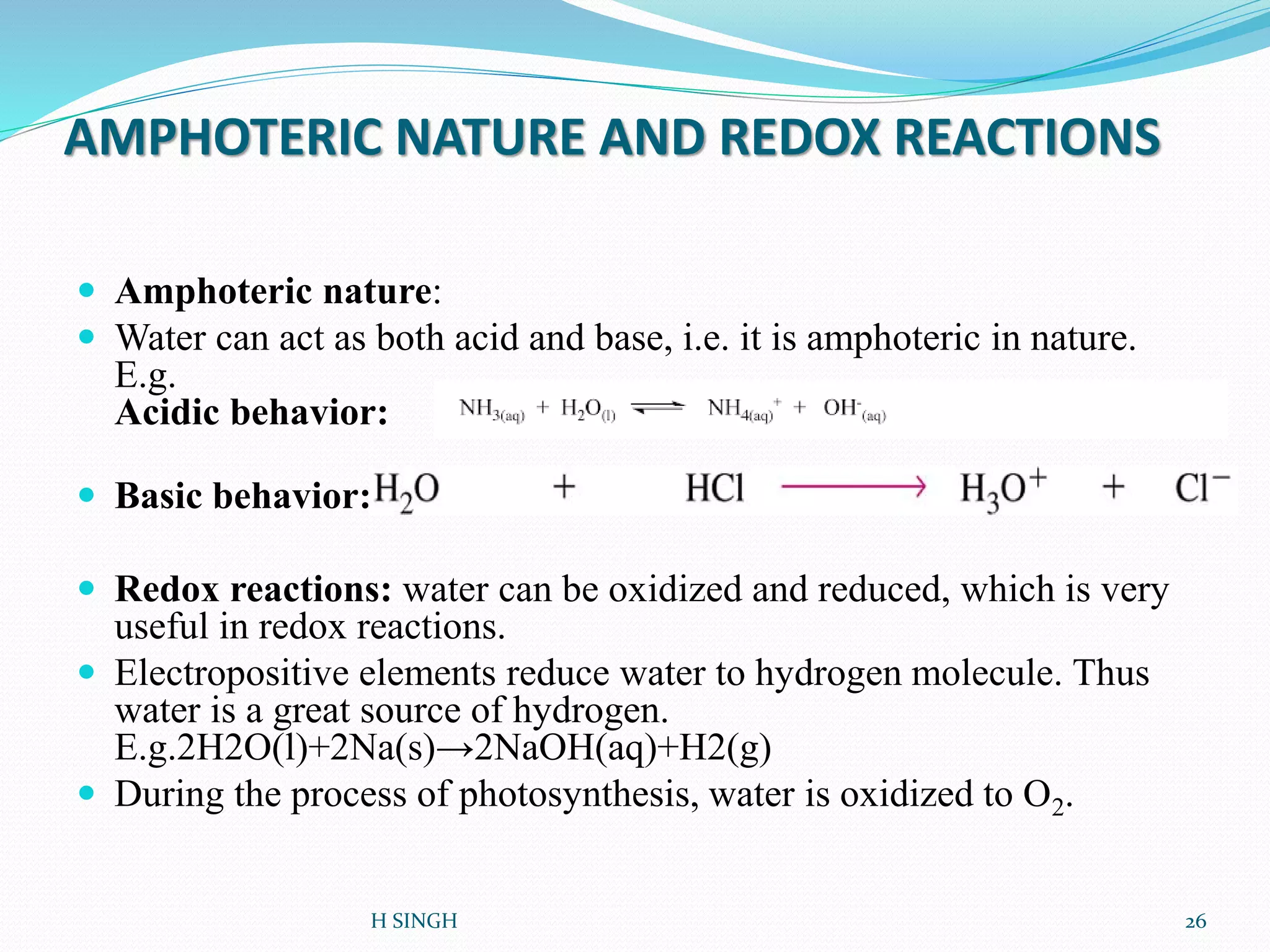
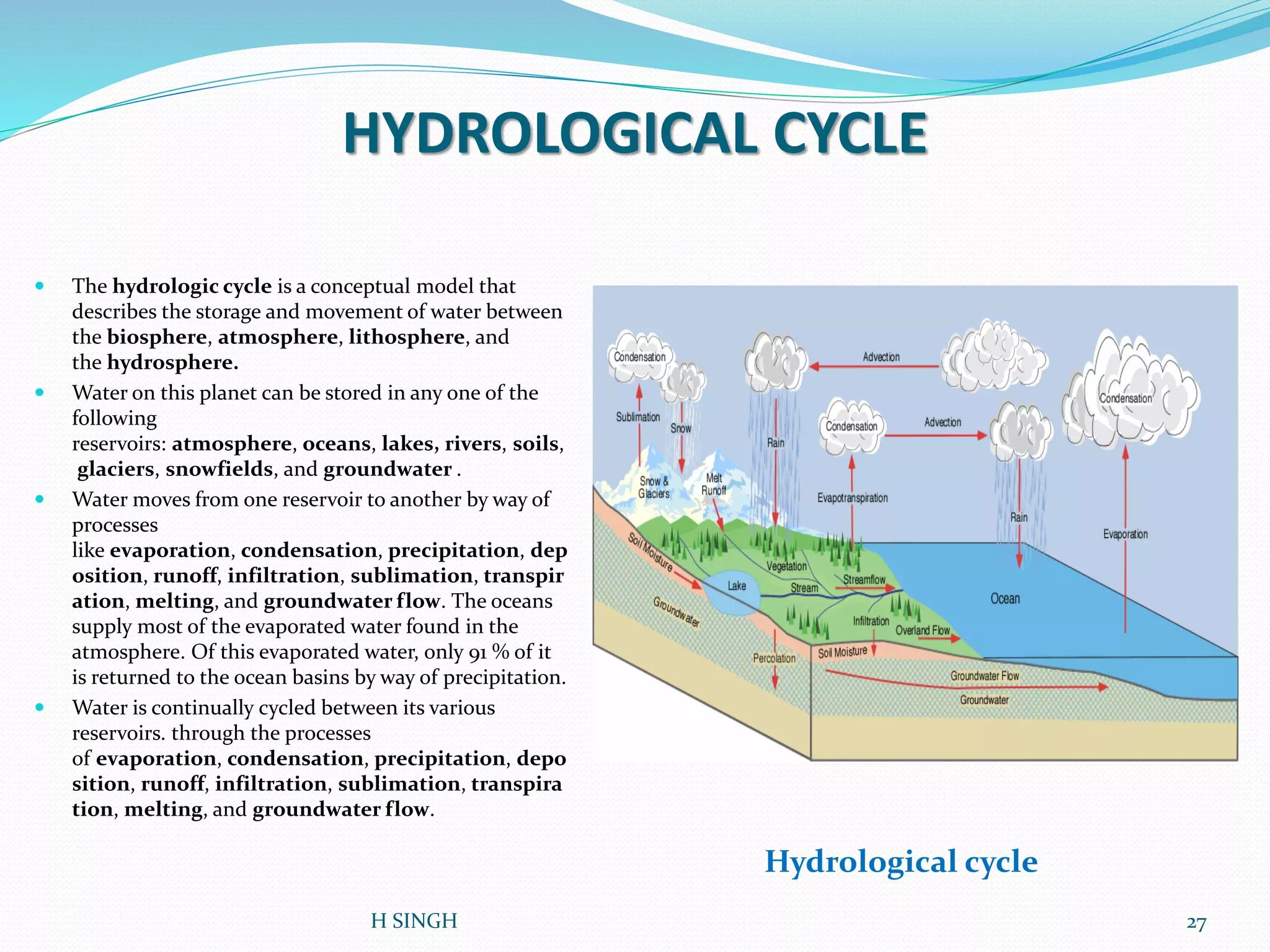
Water is essential for life and has unique properties that make it suitable for life. It exists in nature as a liquid, solid, and gas and has an anomalous property of becoming less dense when frozen. Water's polarity and ability to form hydrogen bonds give it high surface tension, heat capacity, and ability to dissolve many substances, making it a universal solvent. These properties allow water to moderate climate, support biological processes in cells, and drive the water cycle which is crucial for life on Earth.