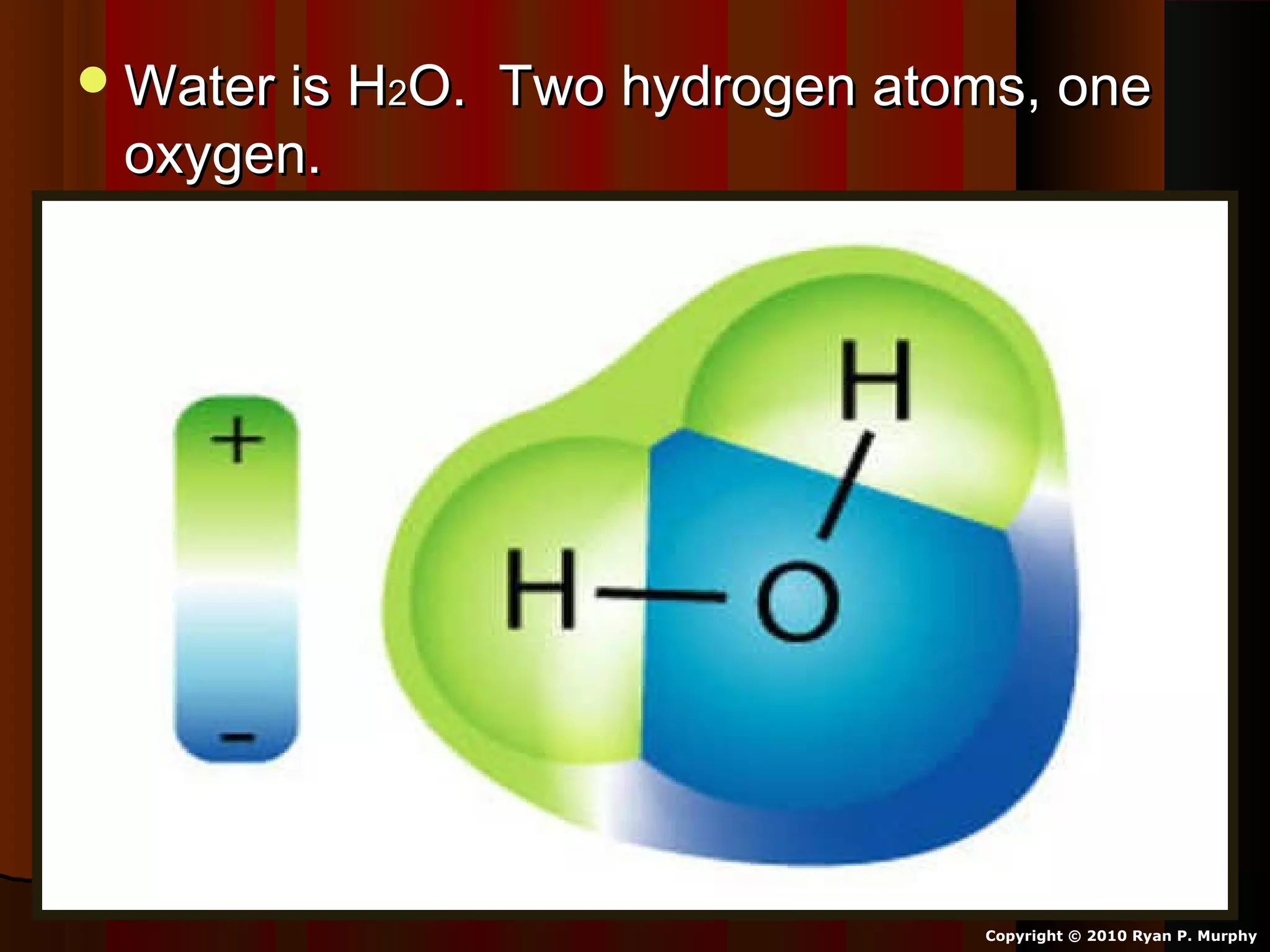
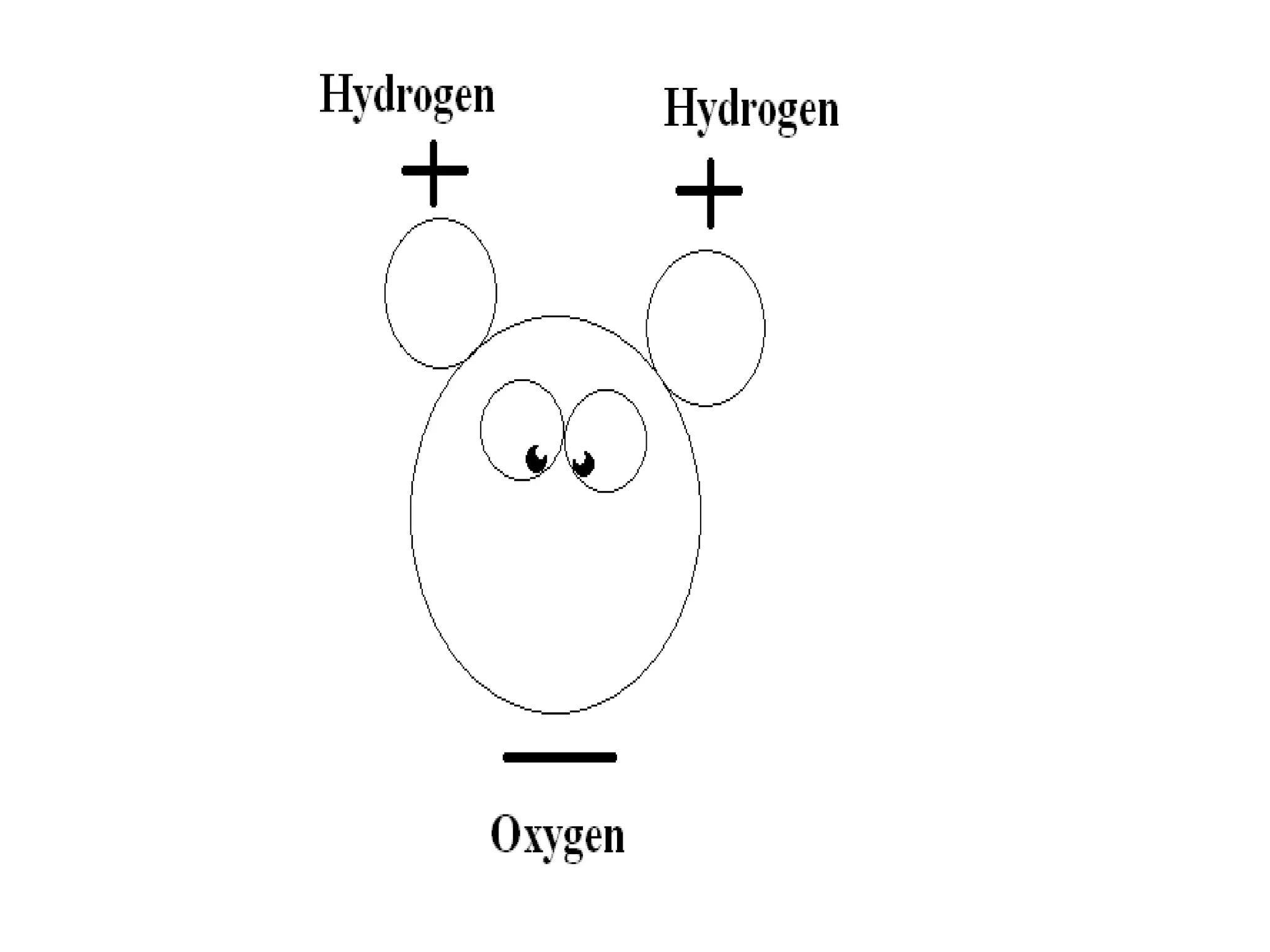
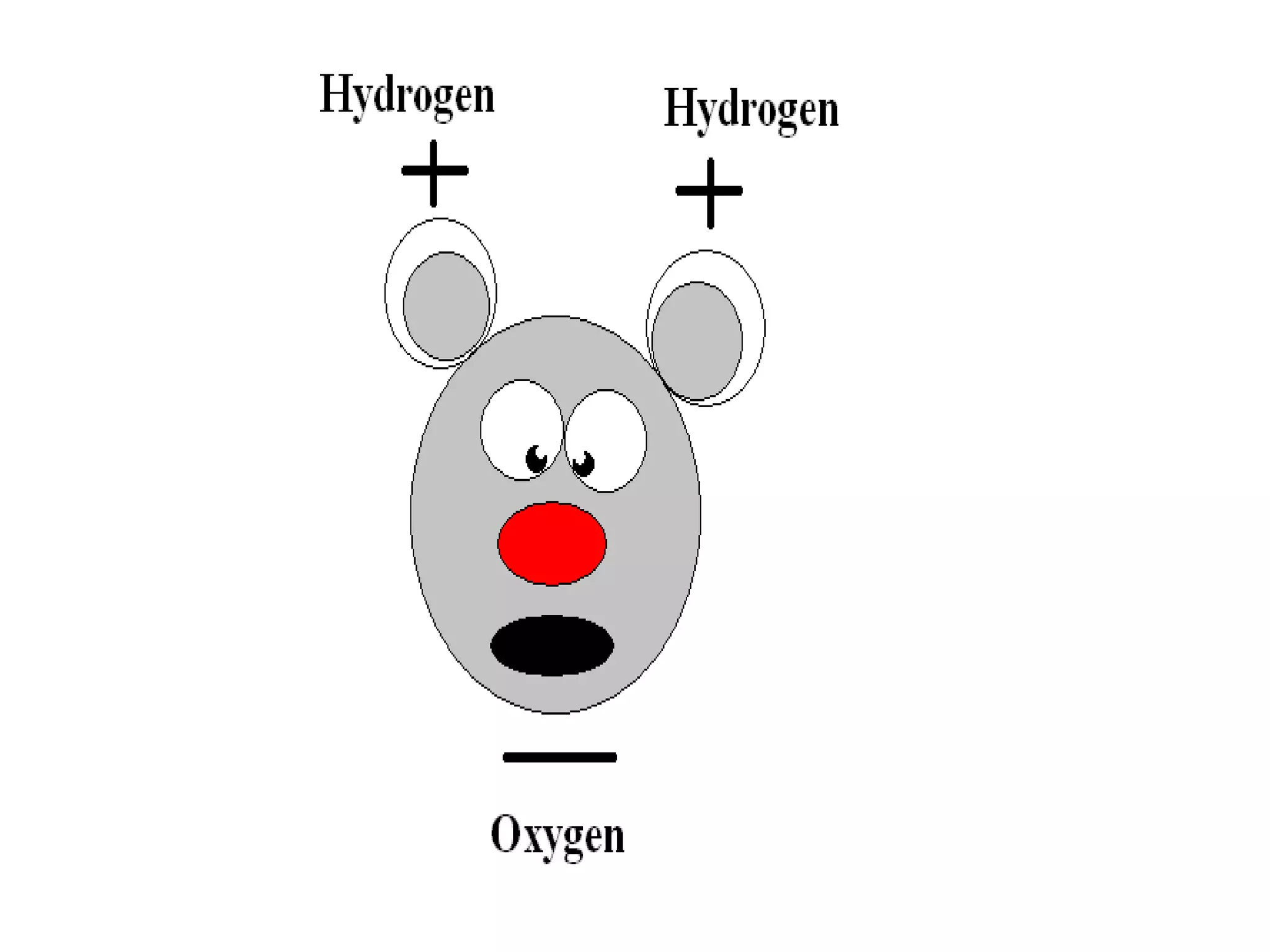
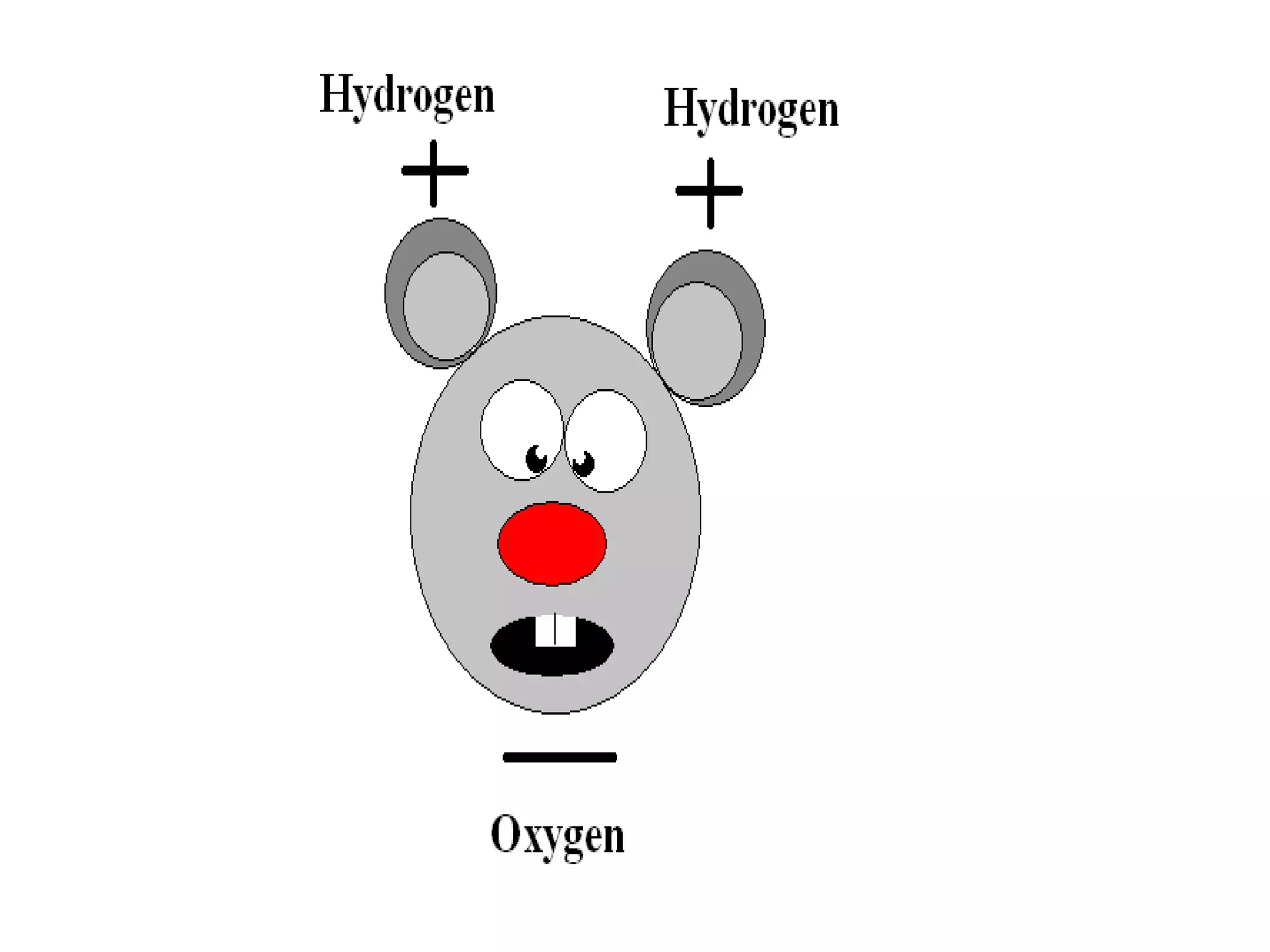
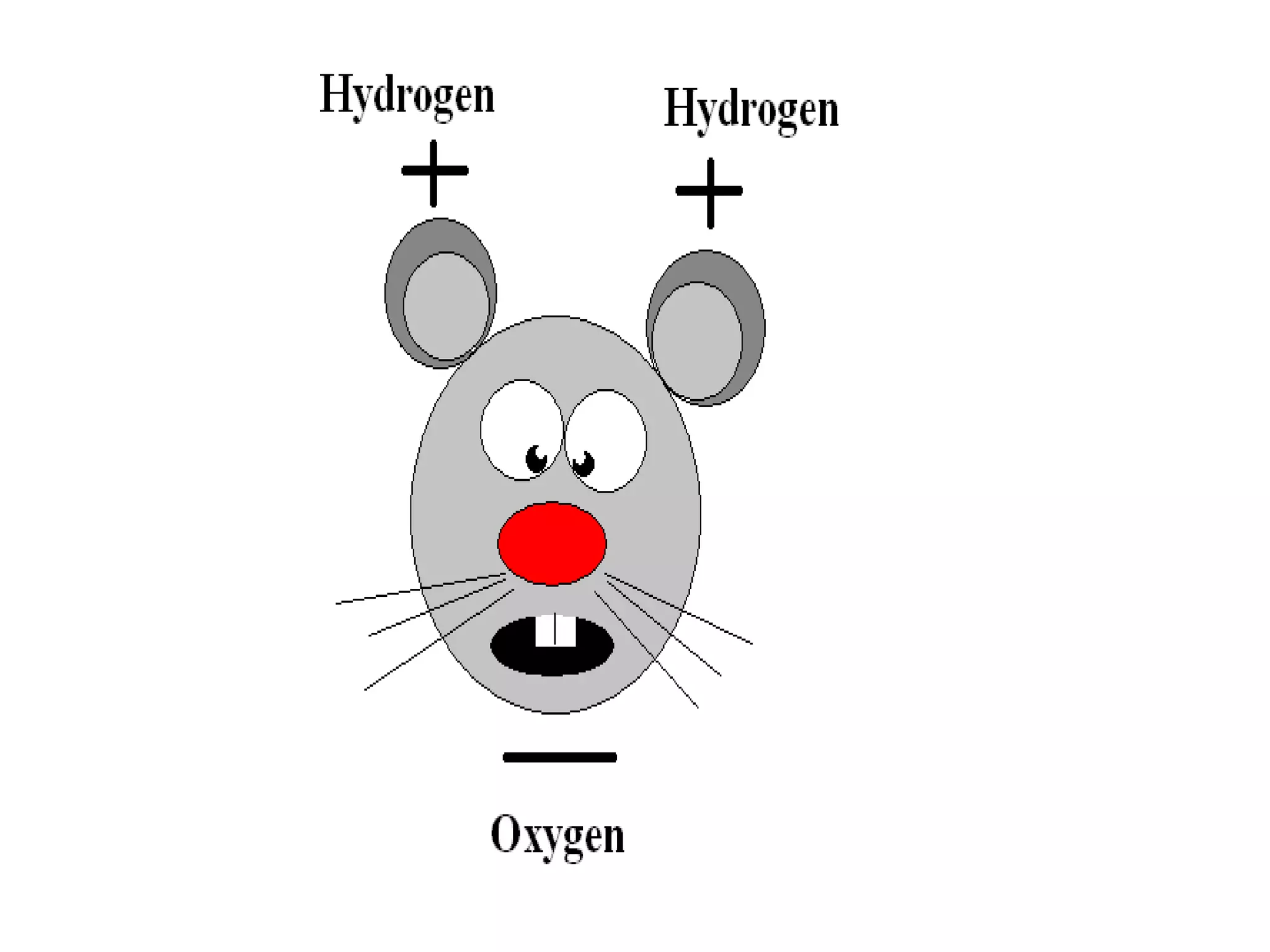
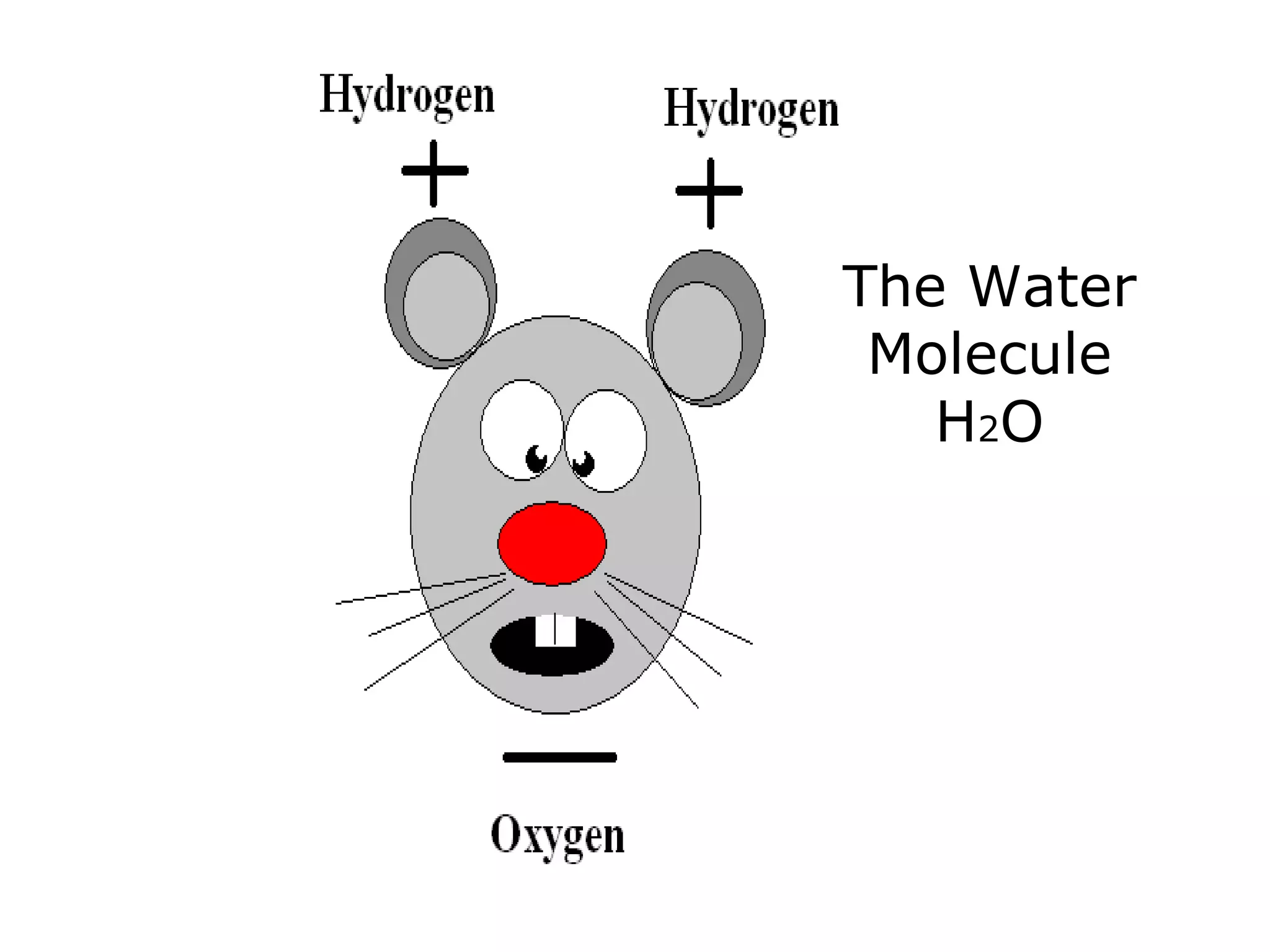
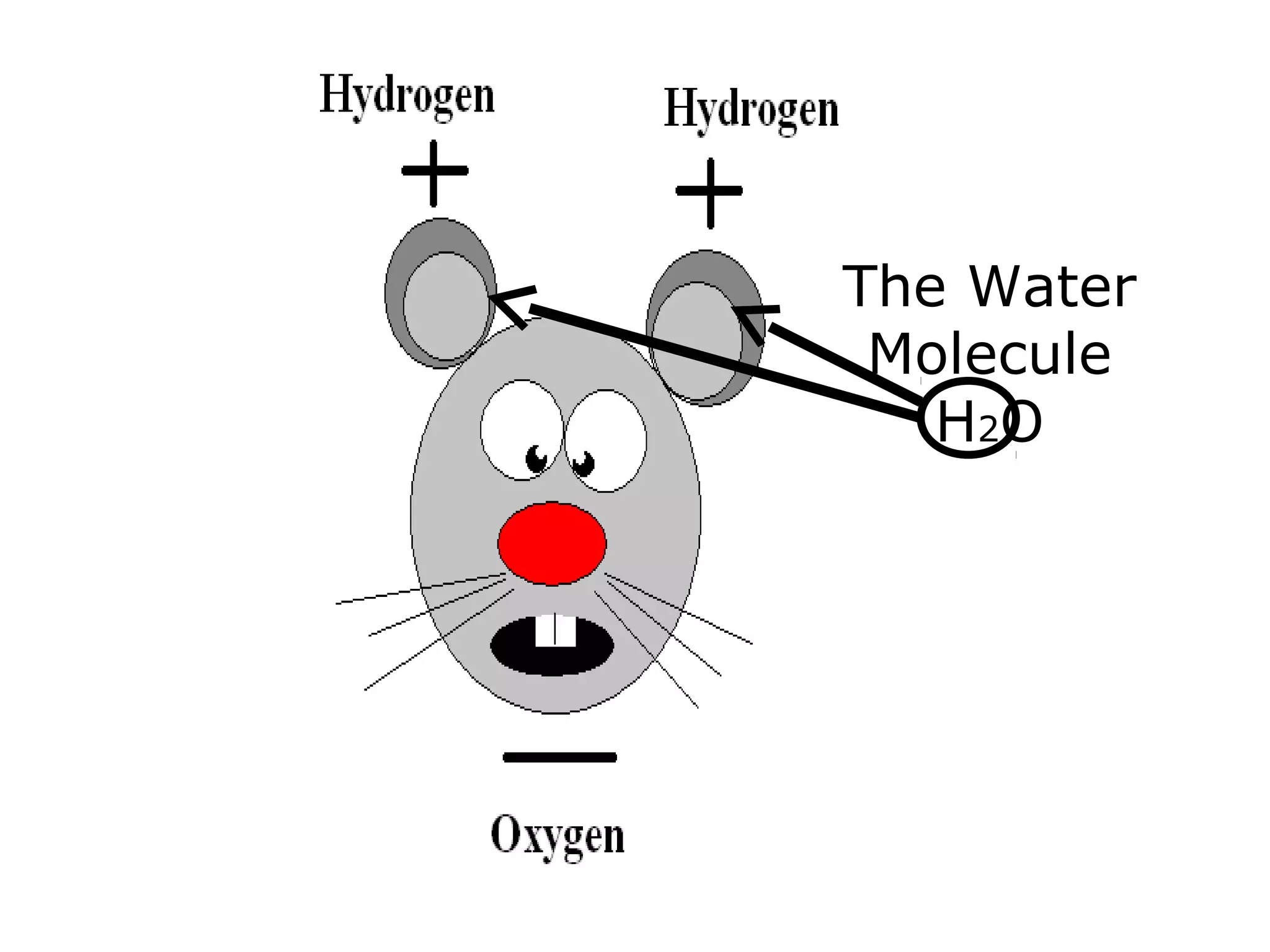
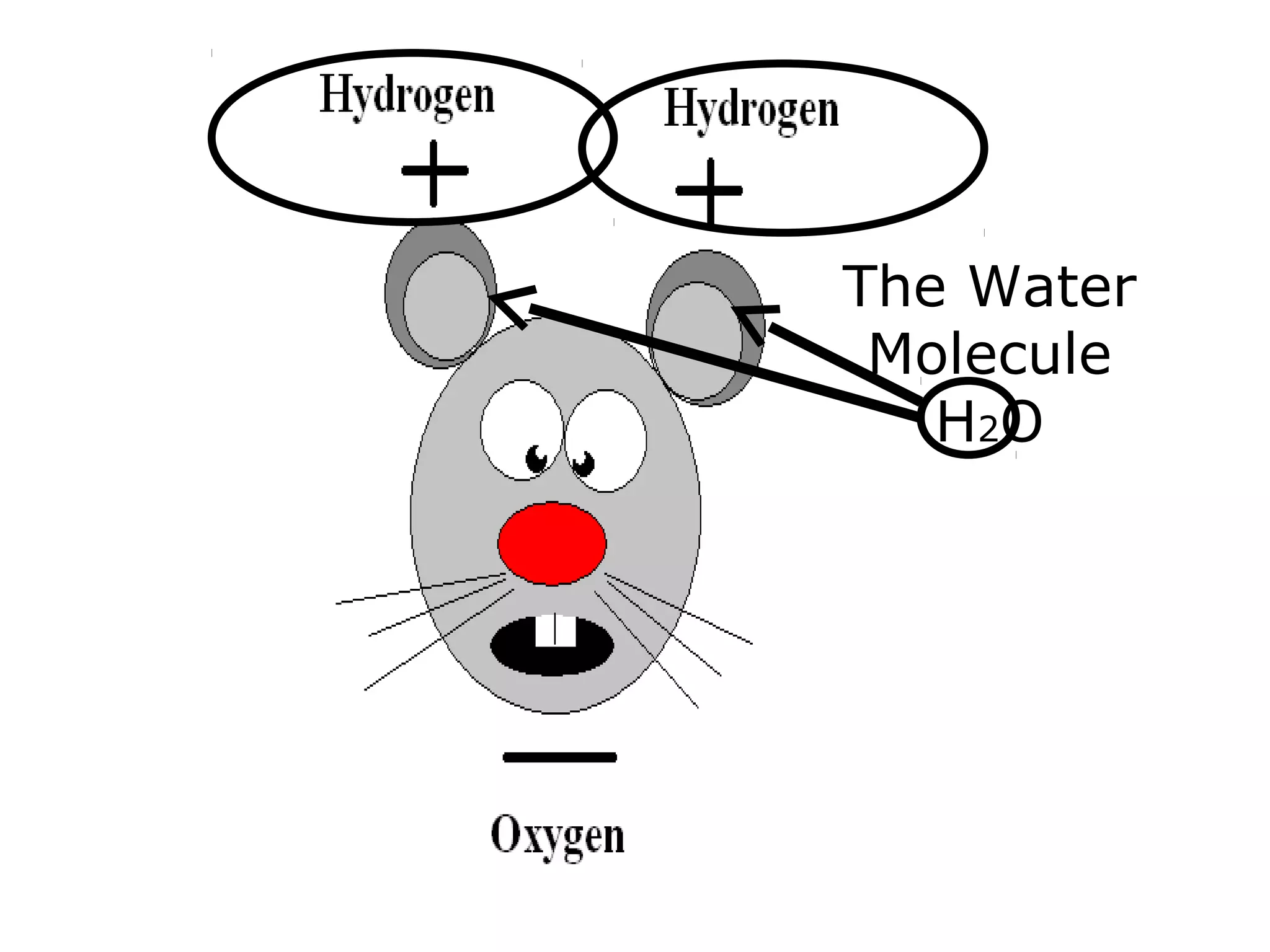
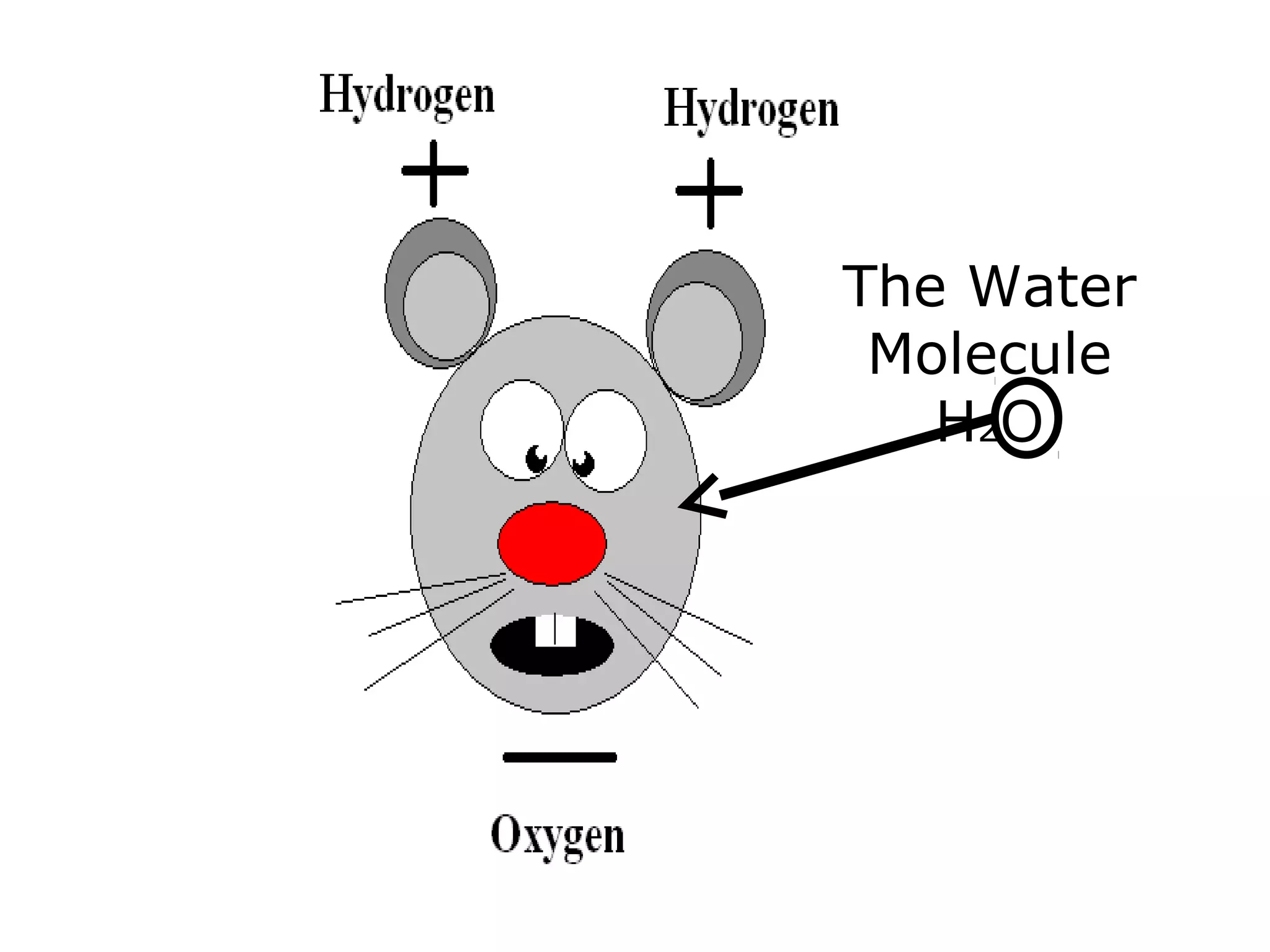
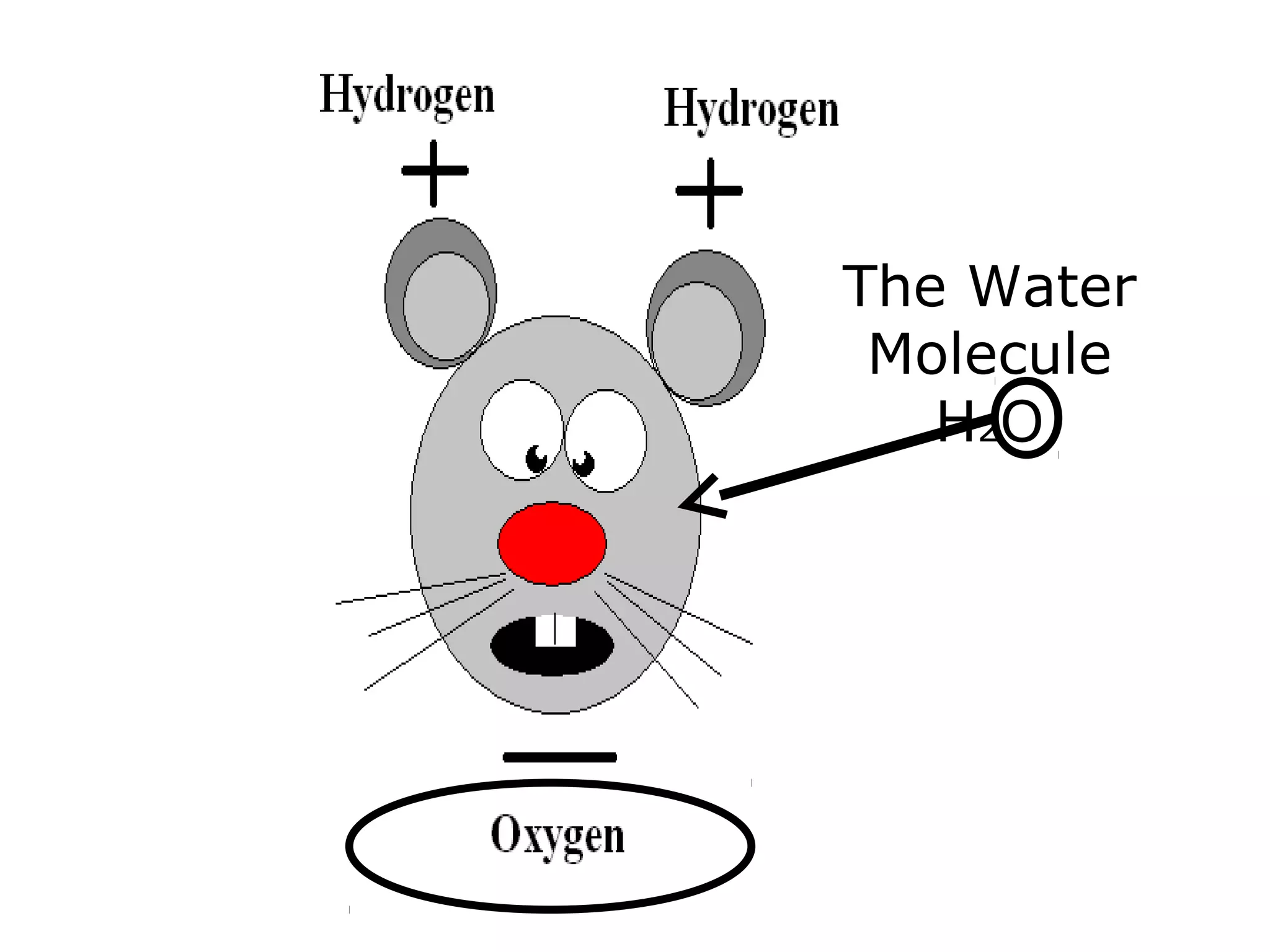
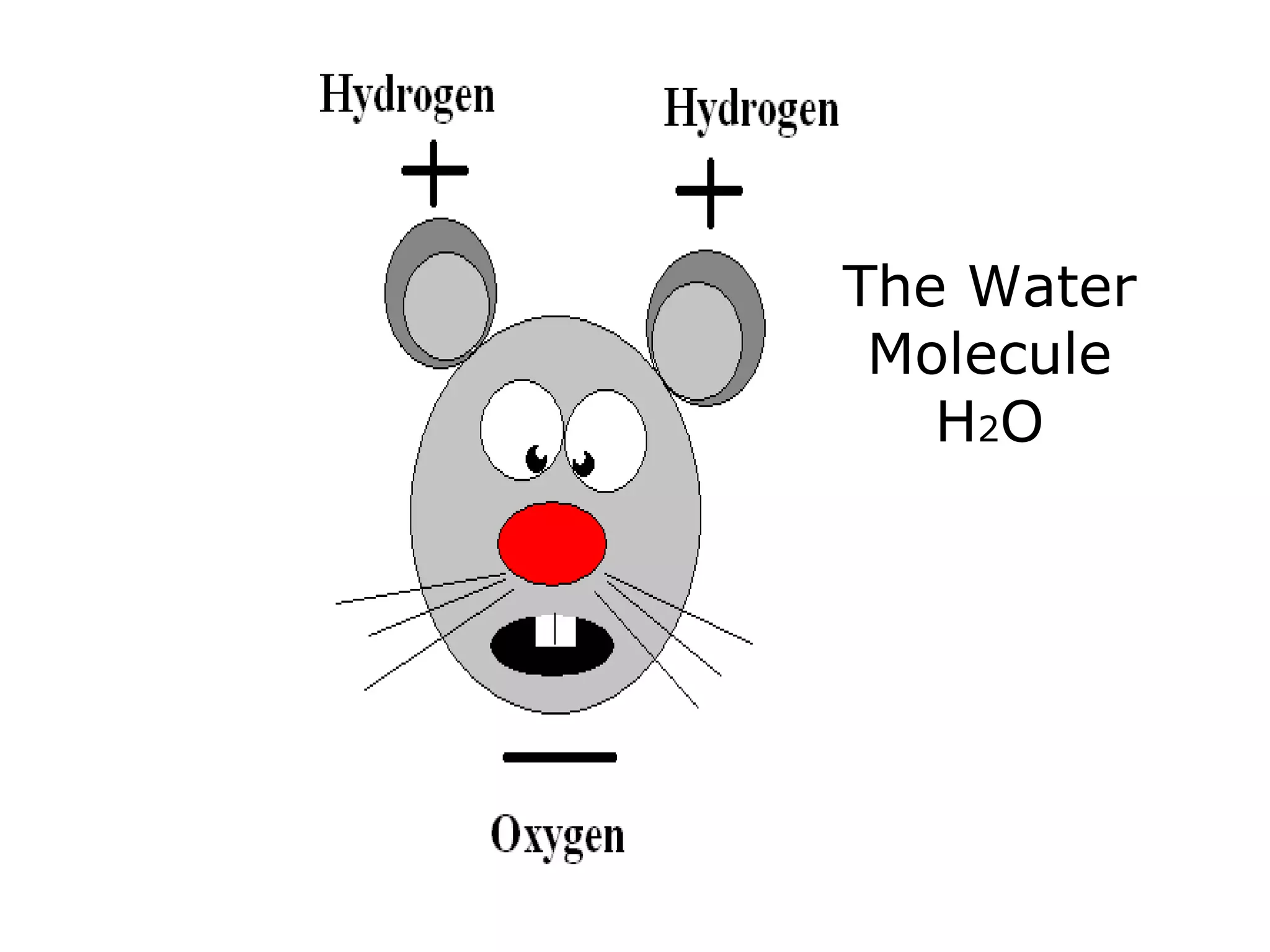
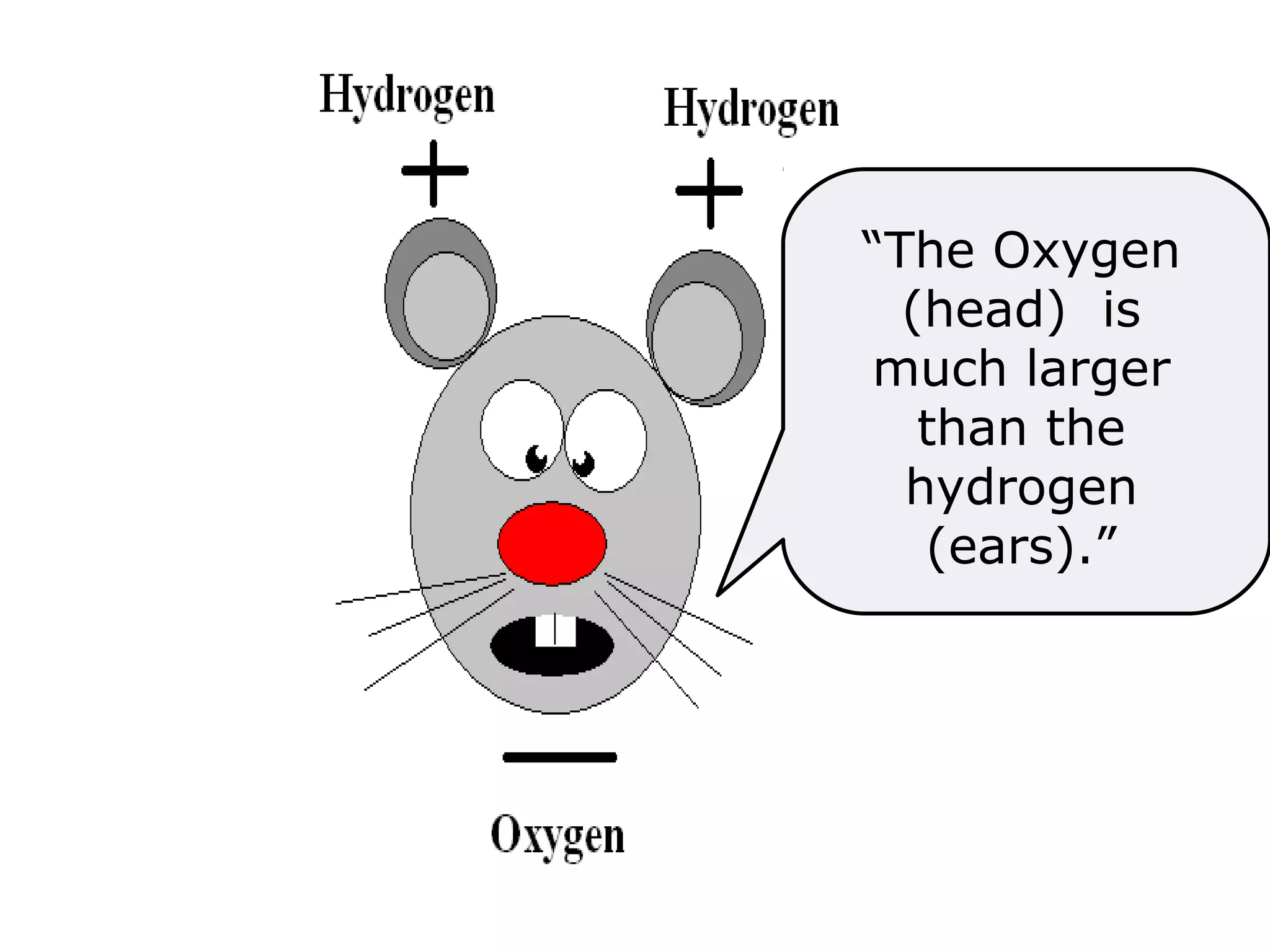
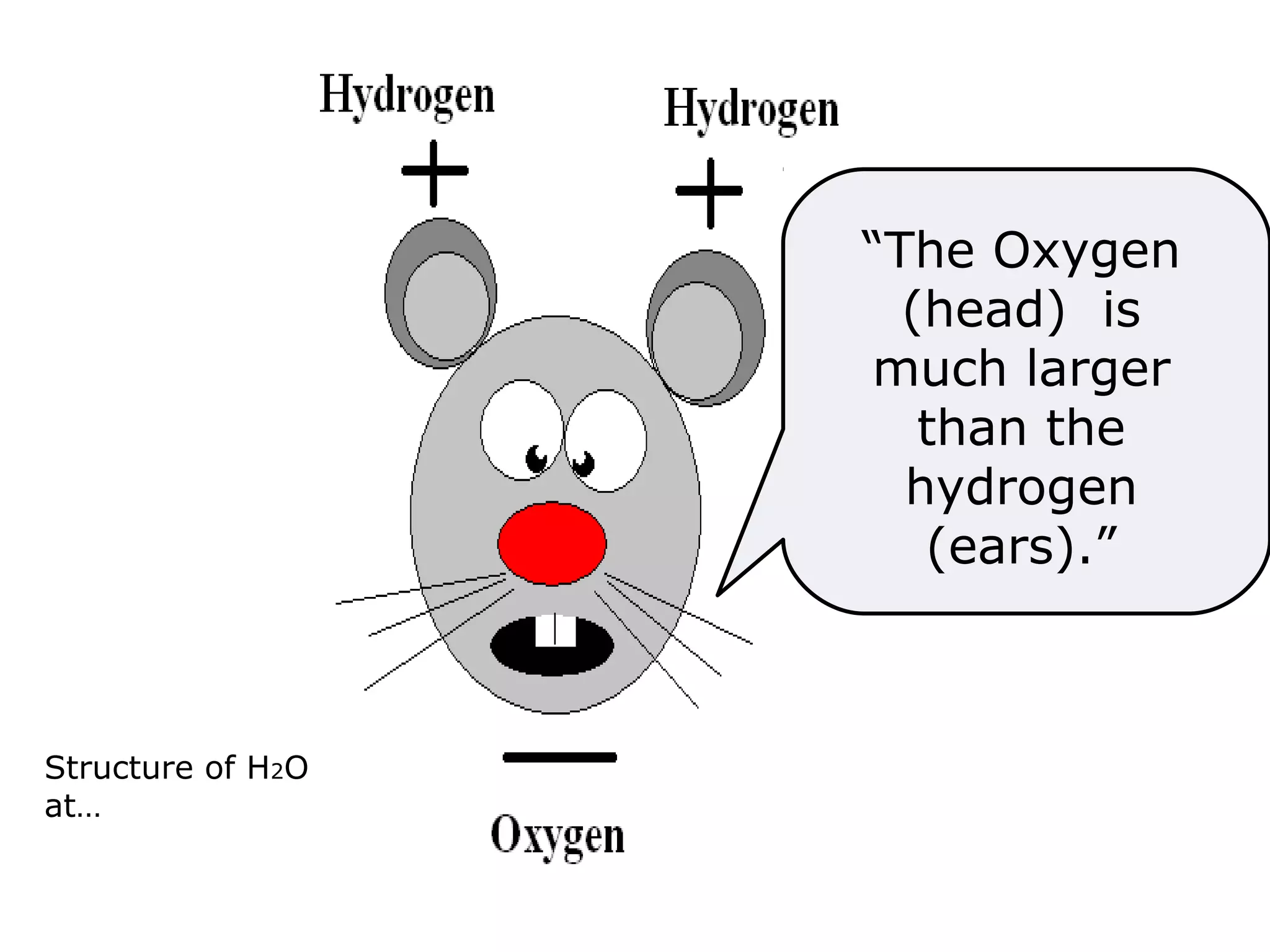
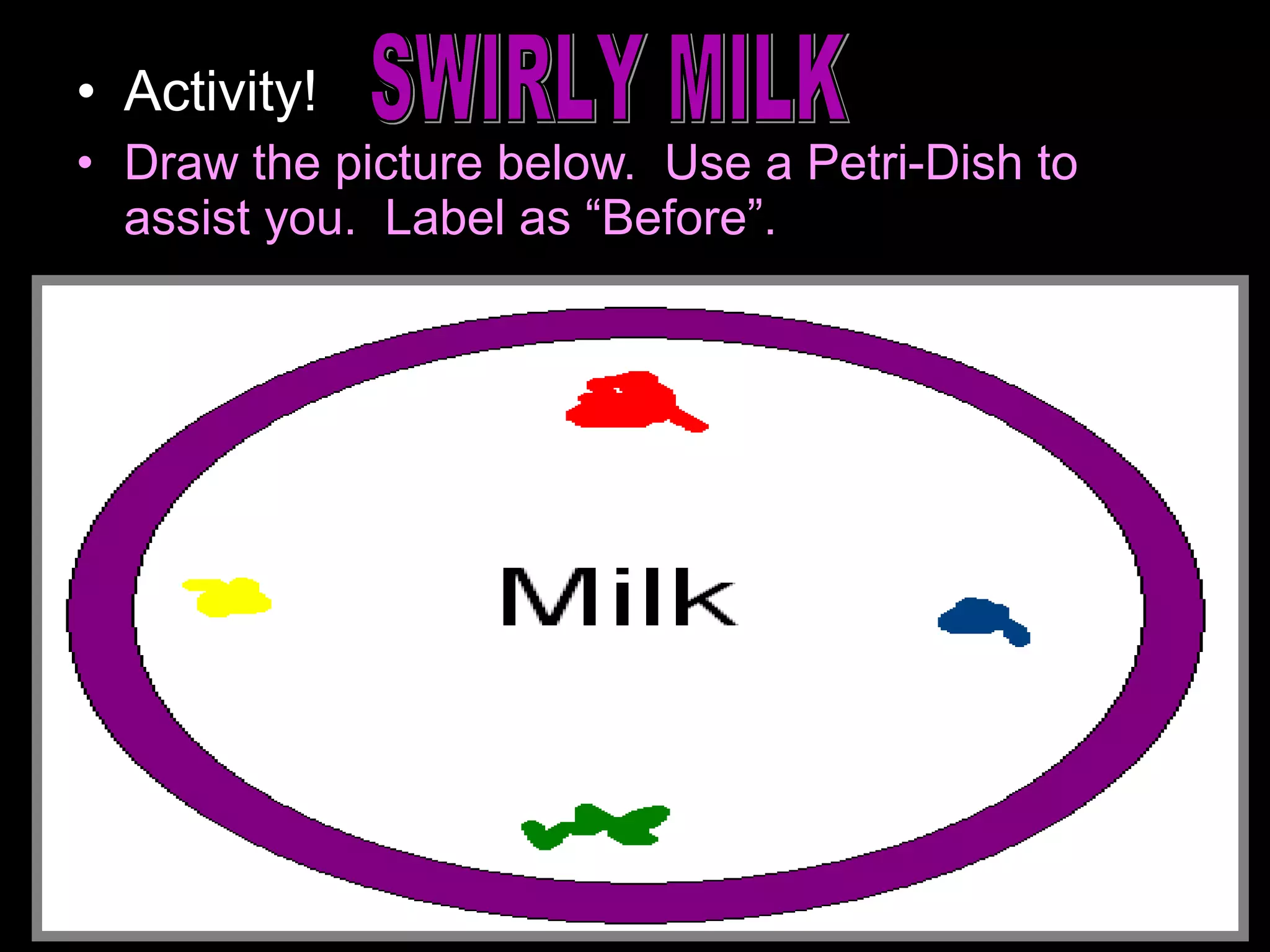
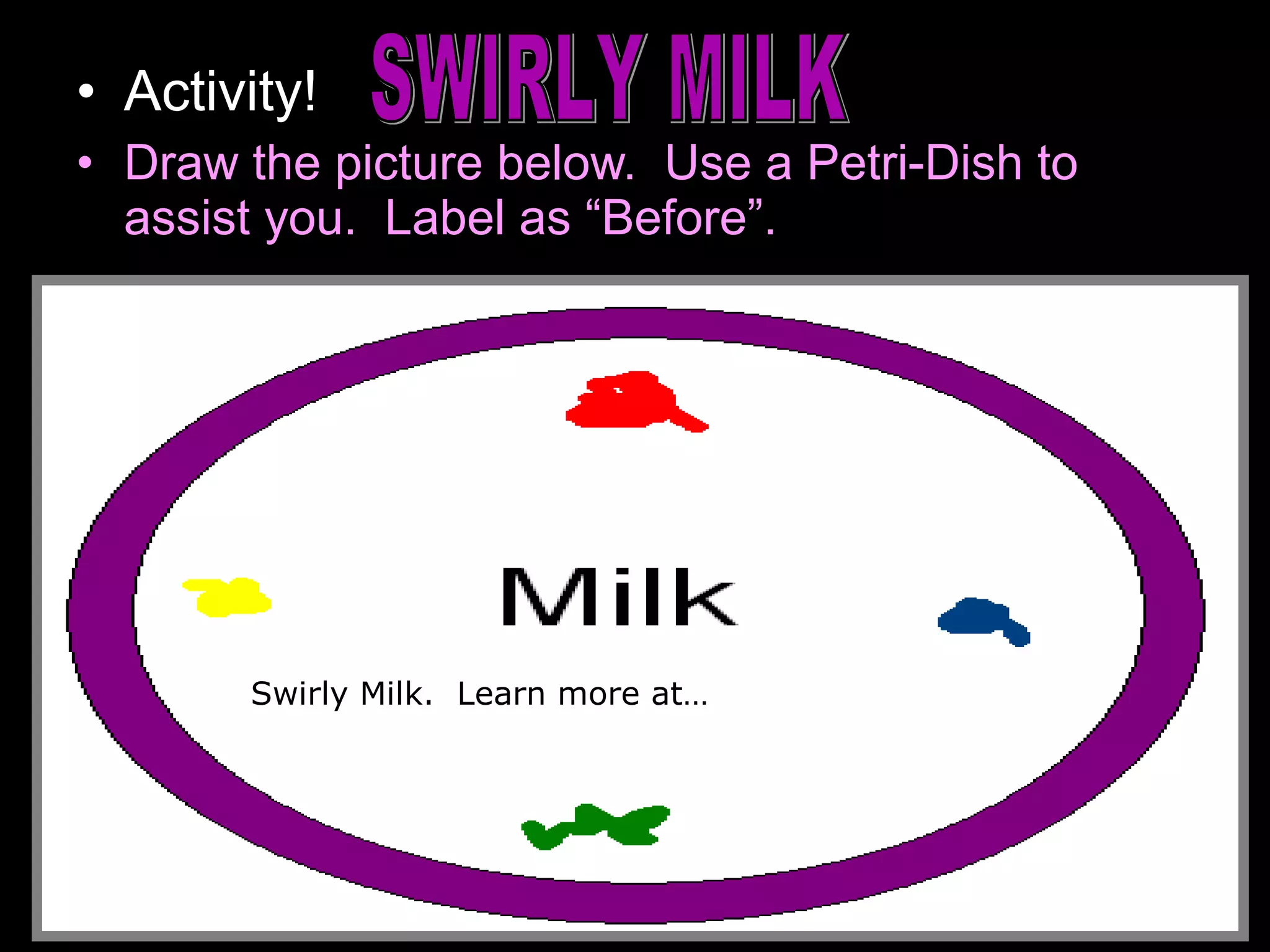
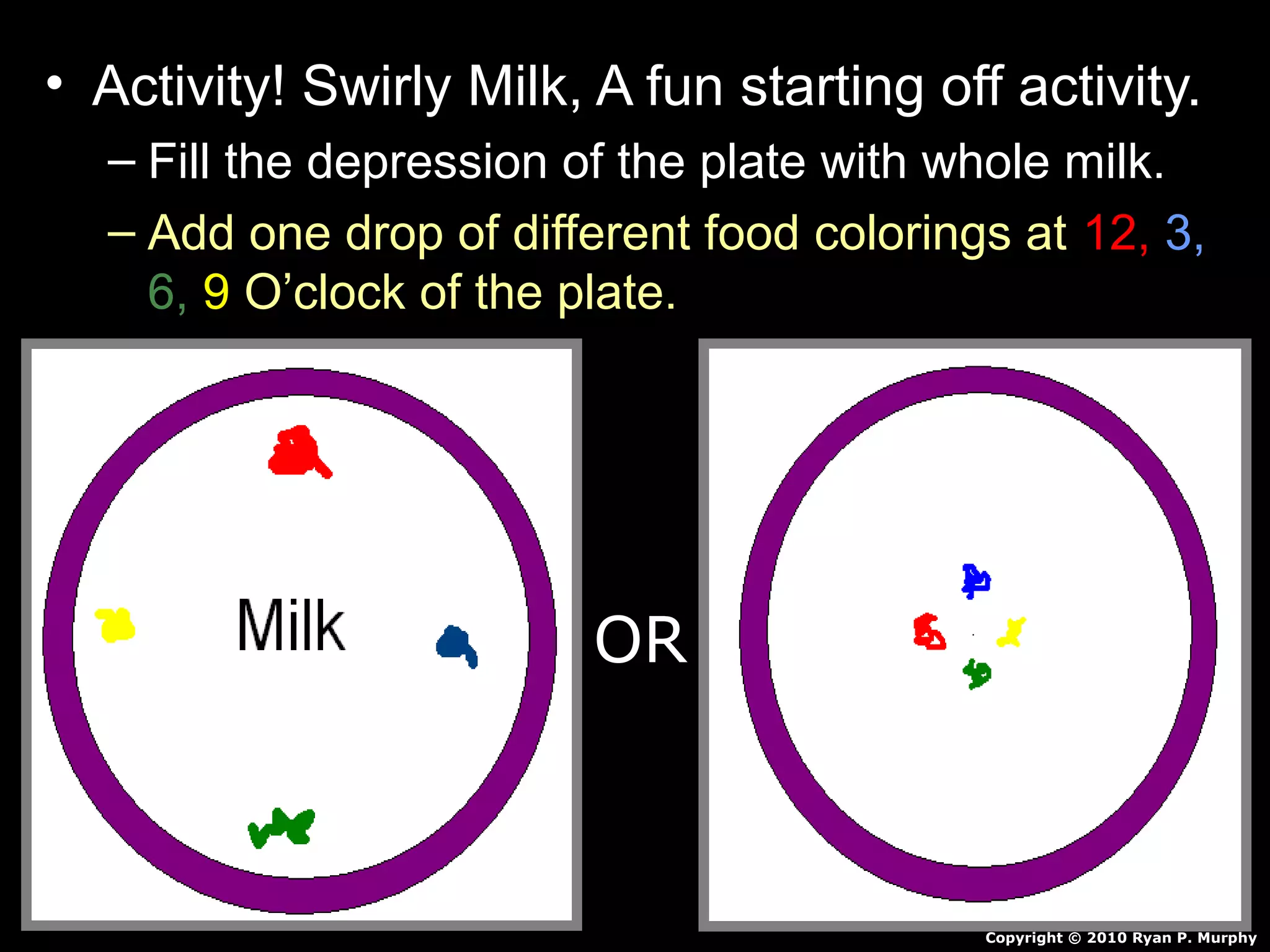
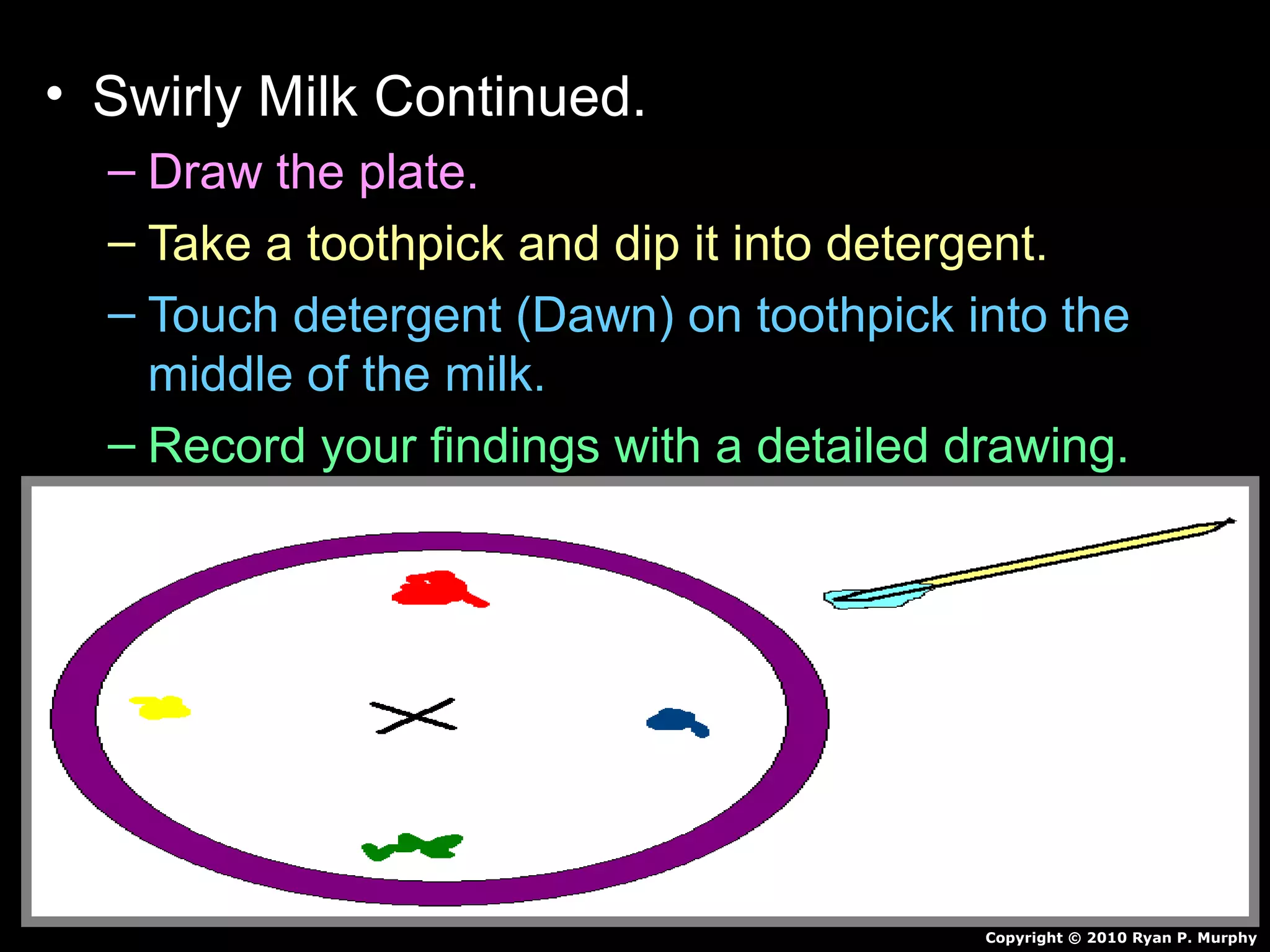
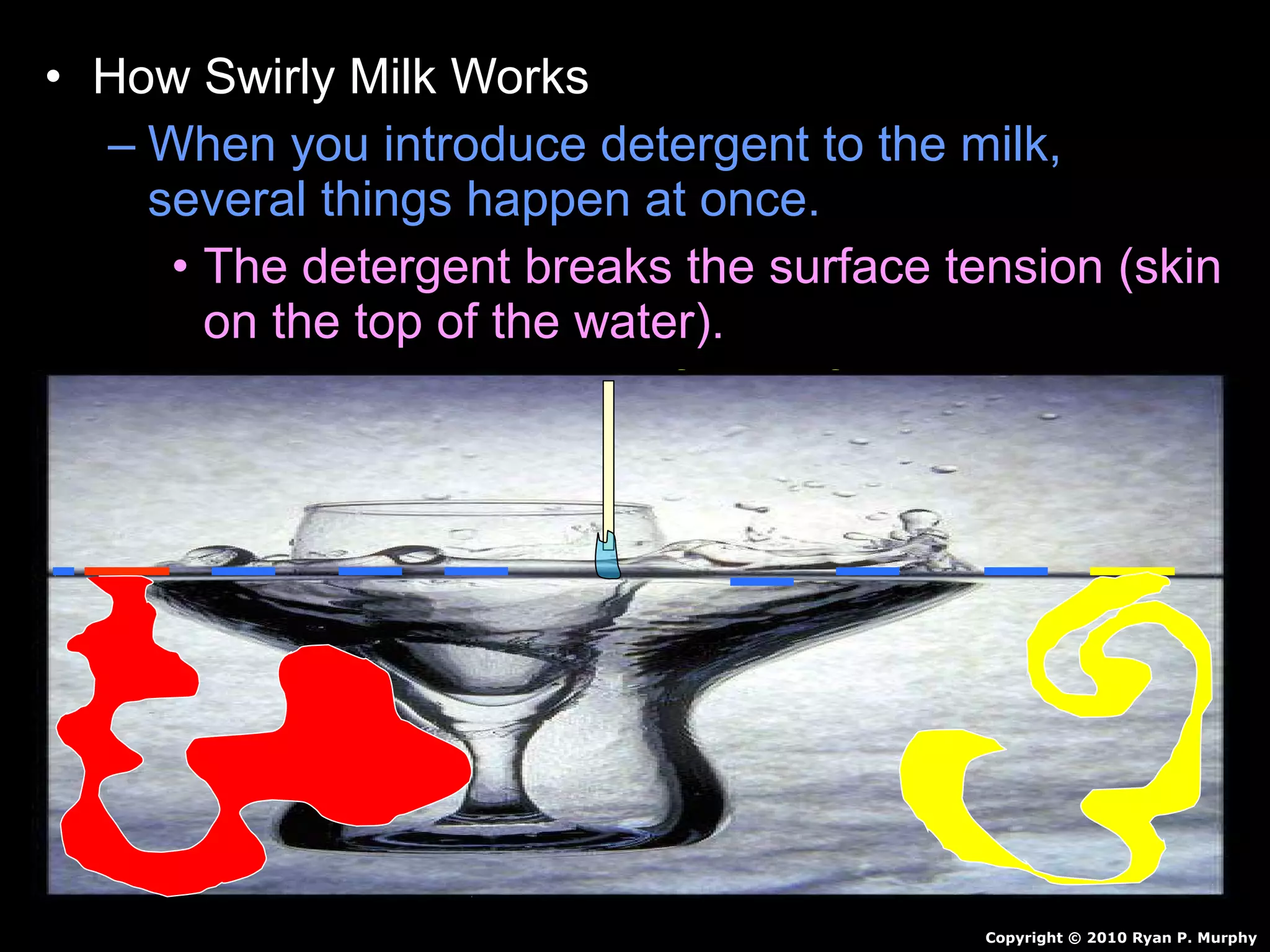
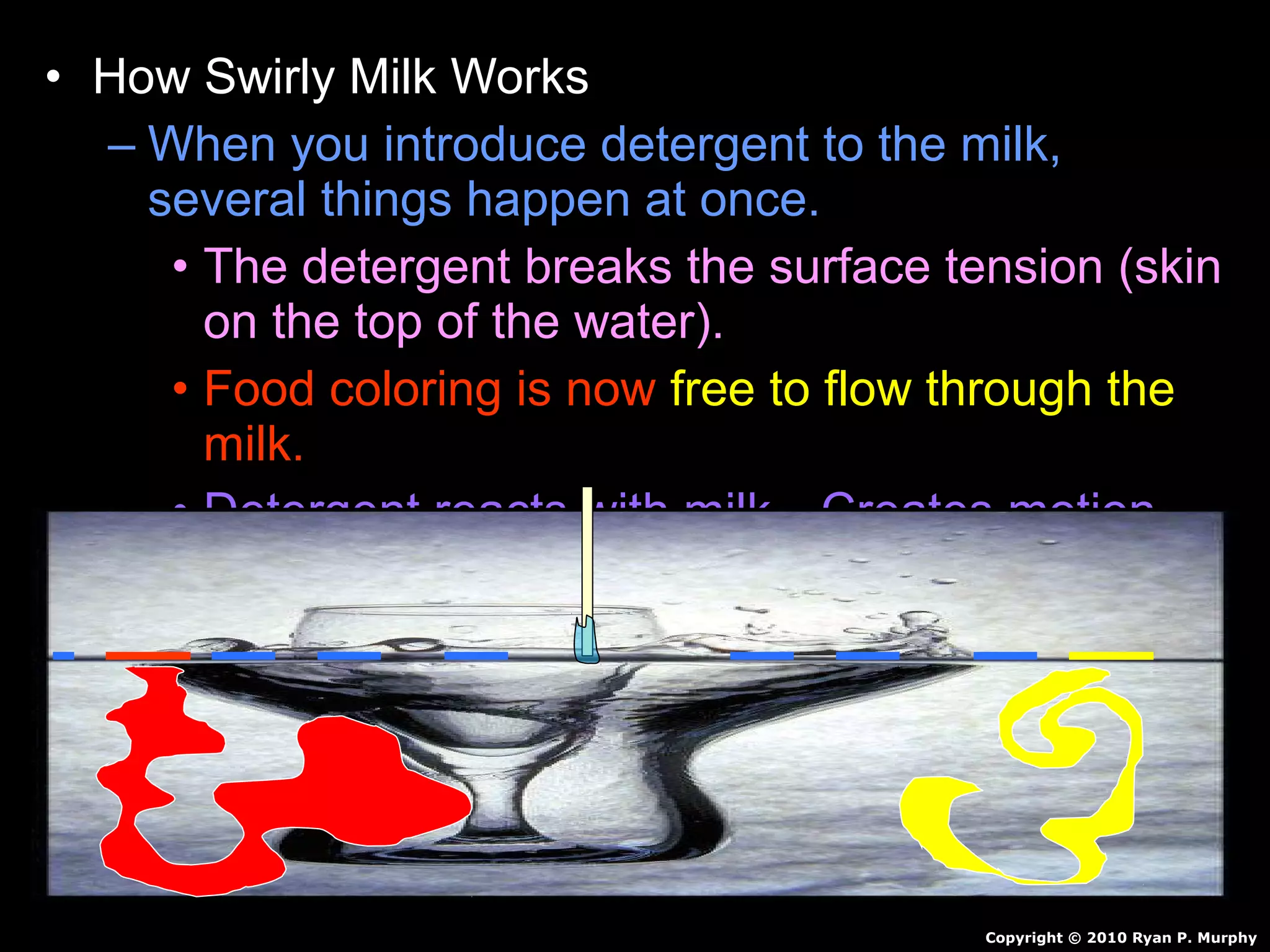
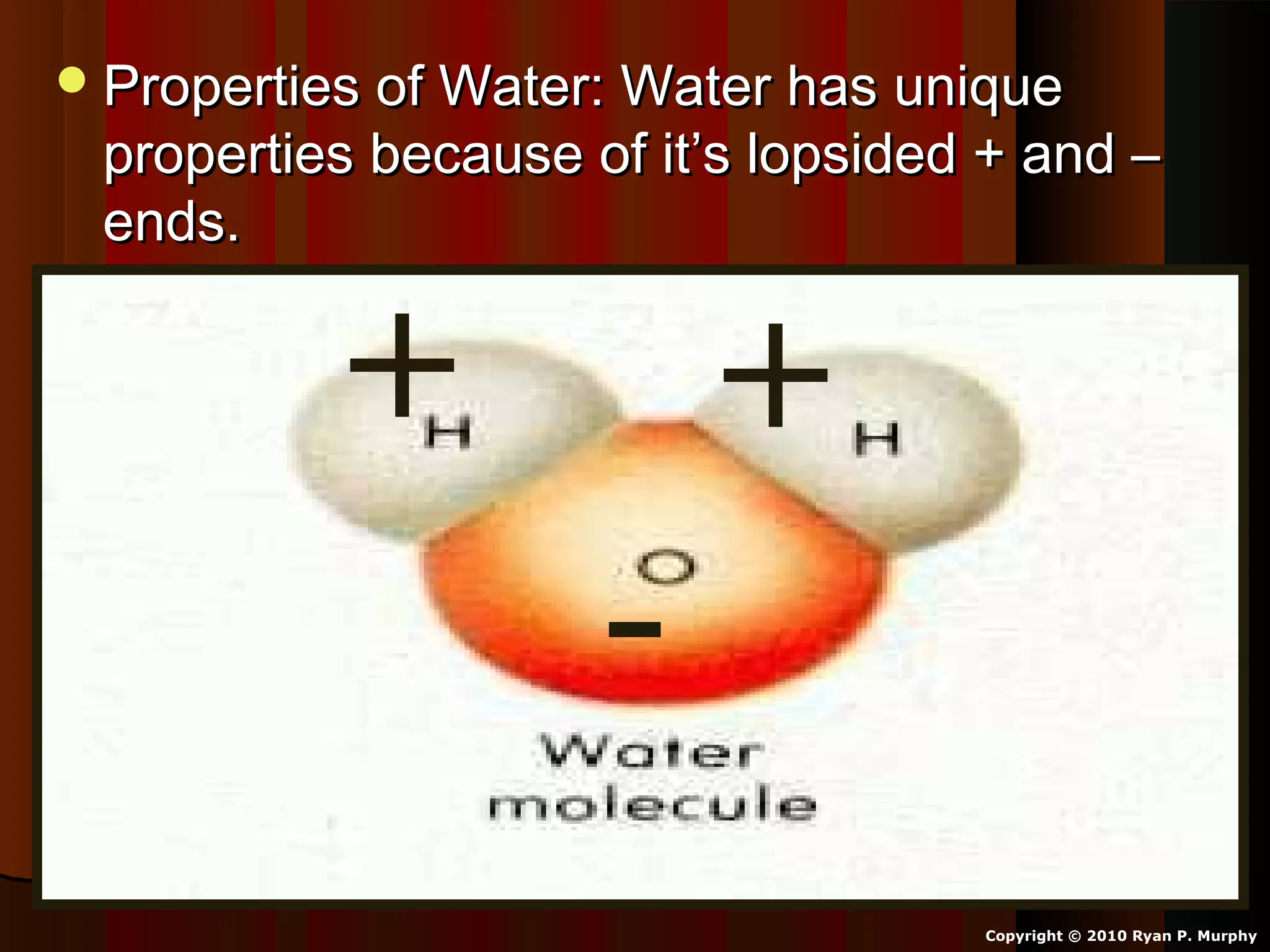
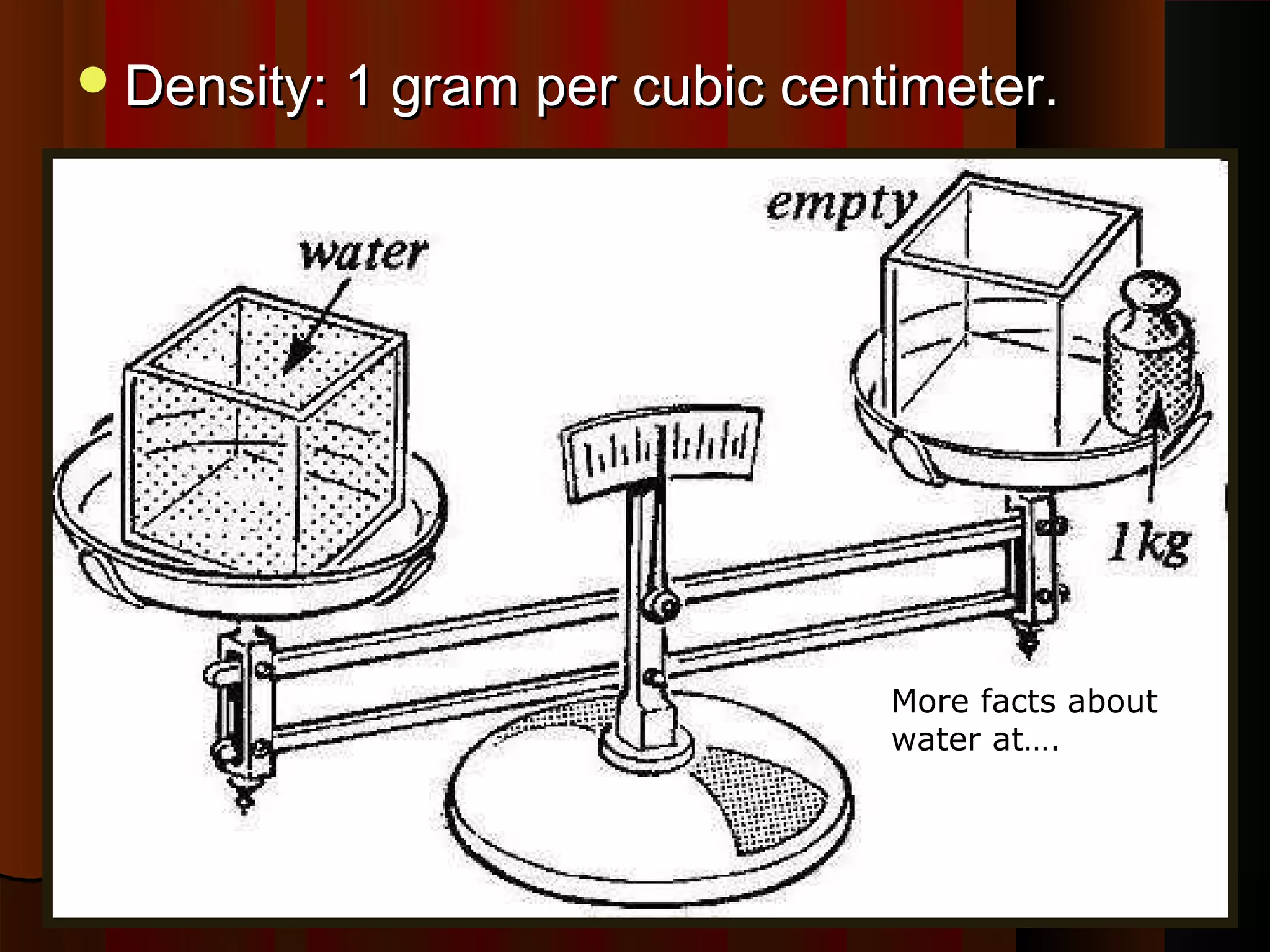
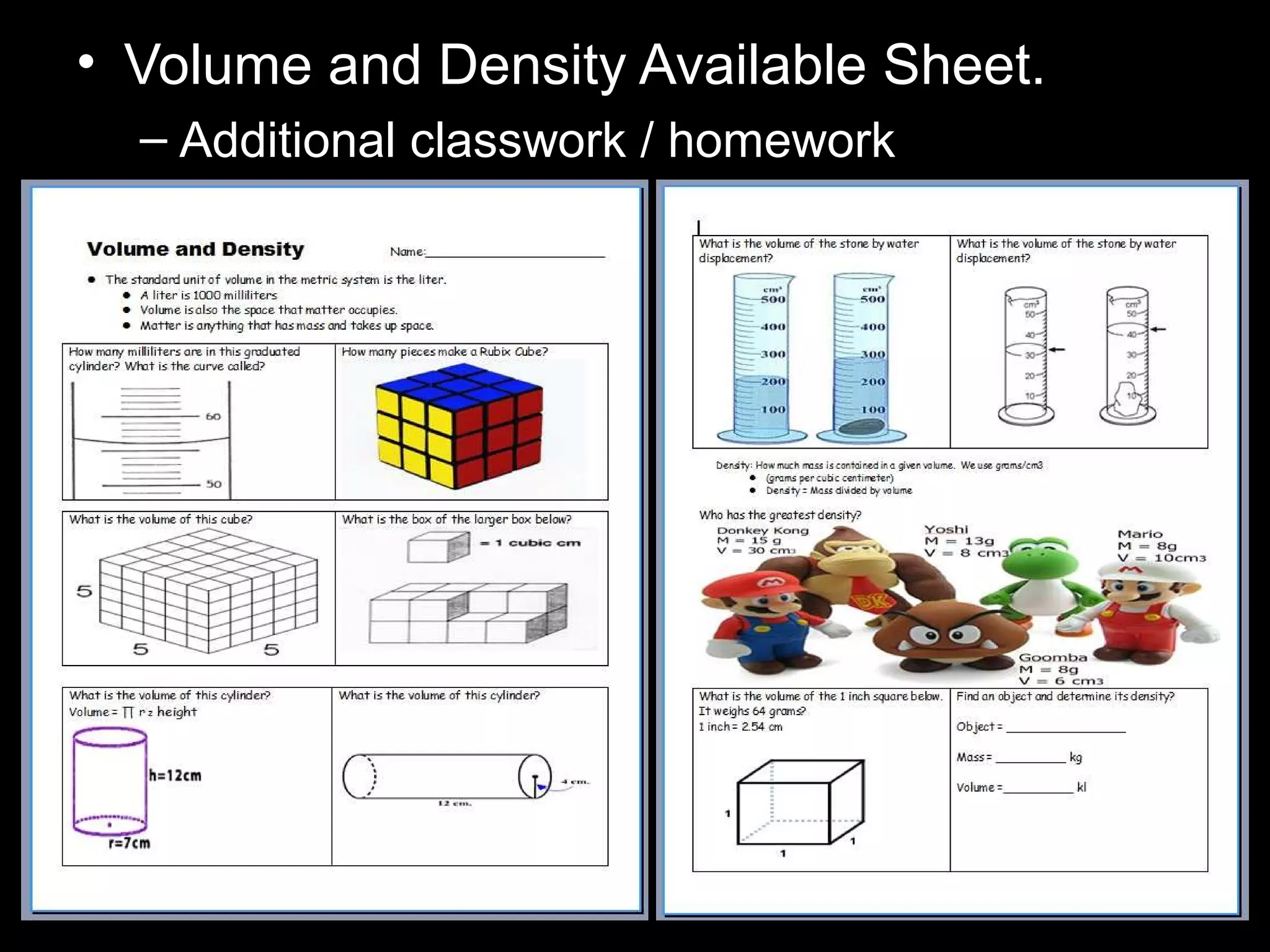
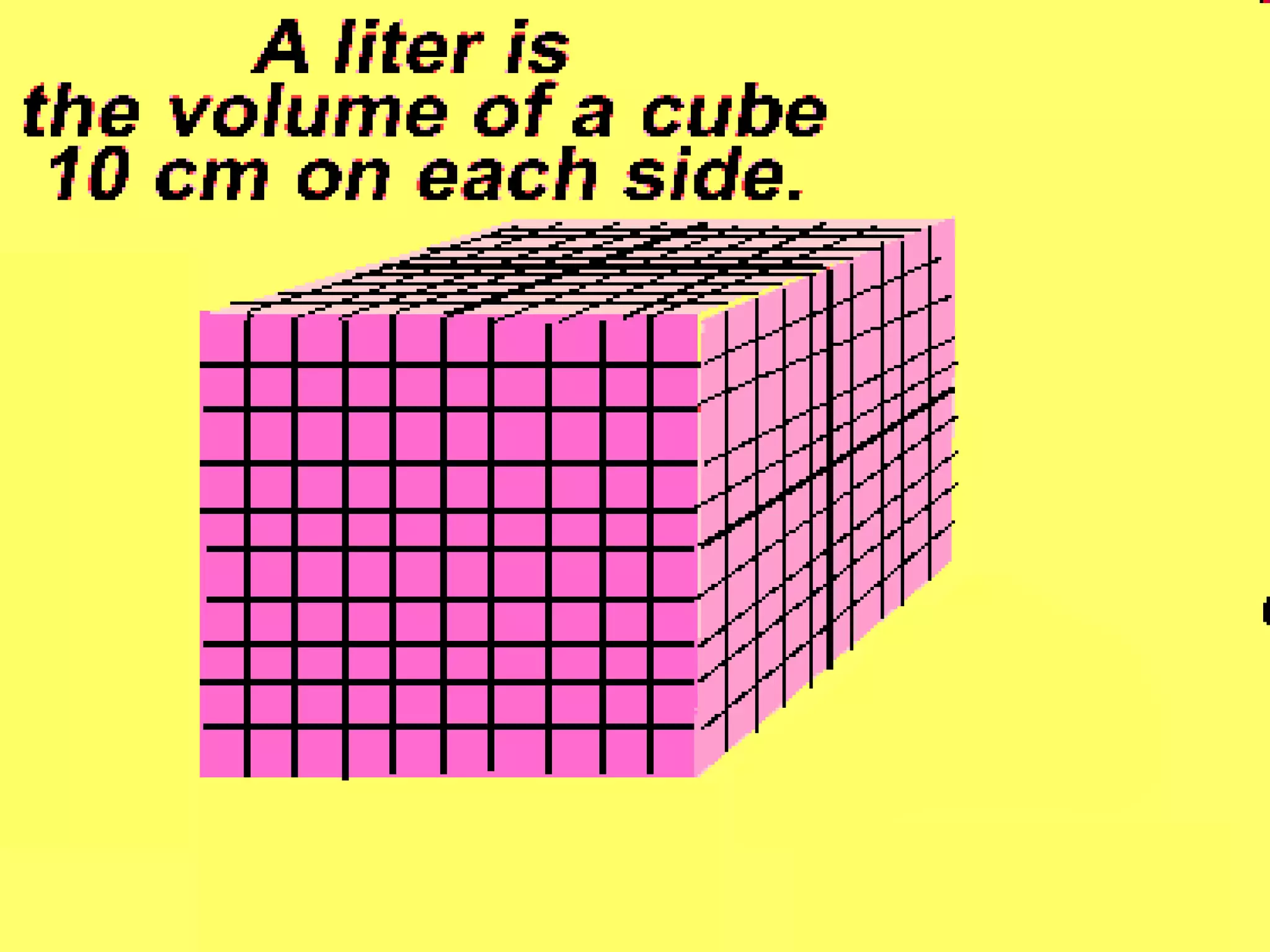
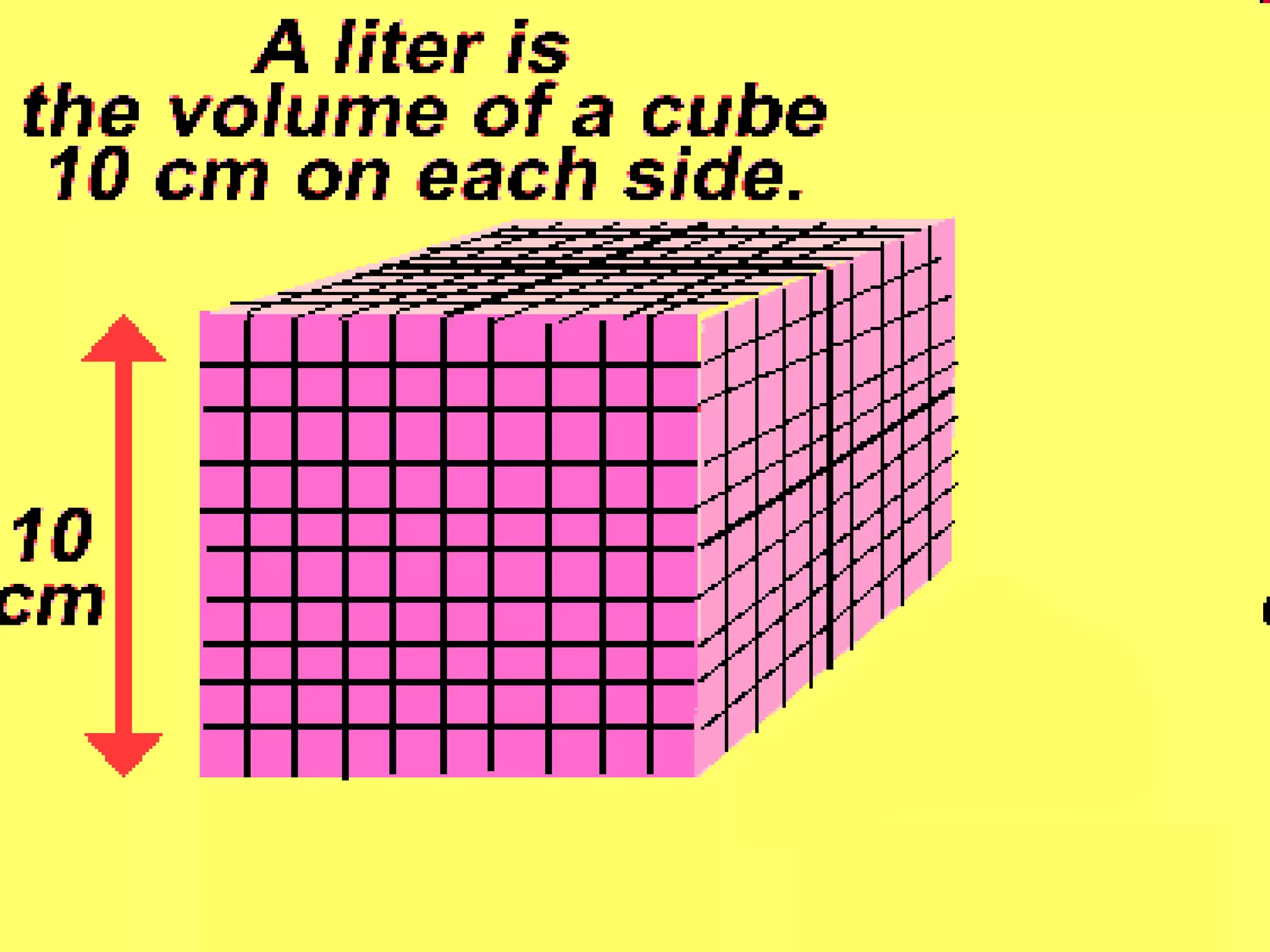
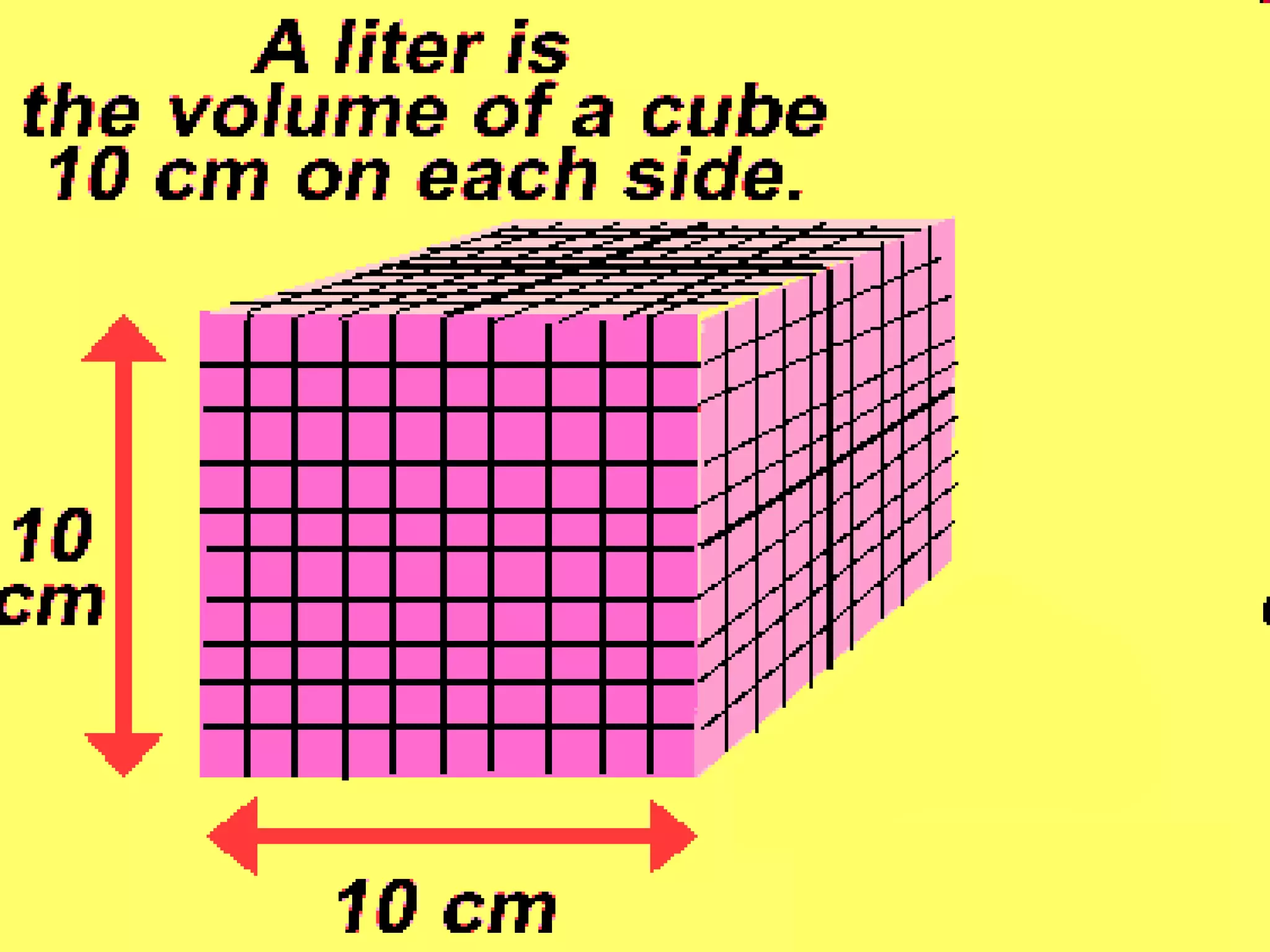
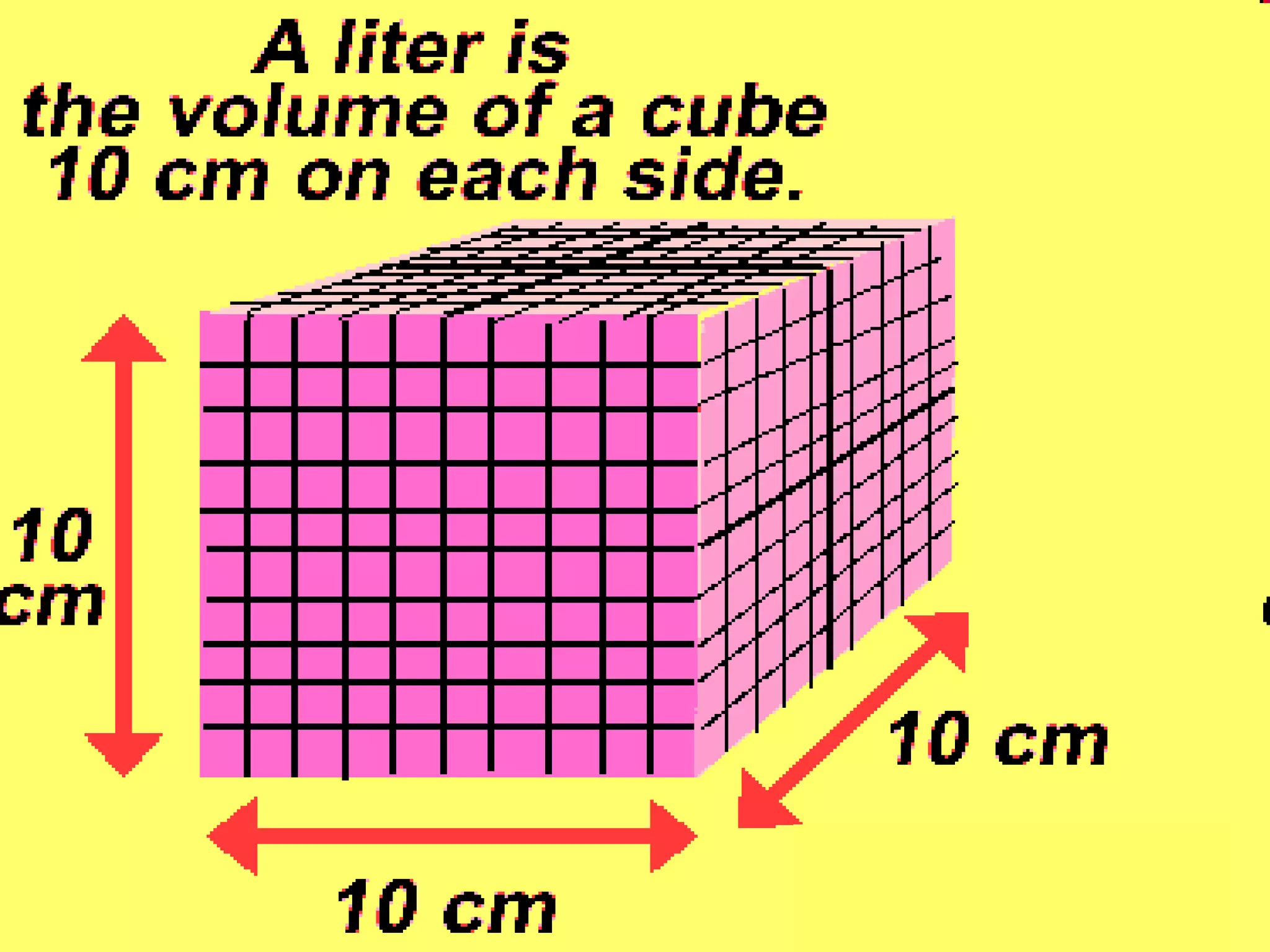
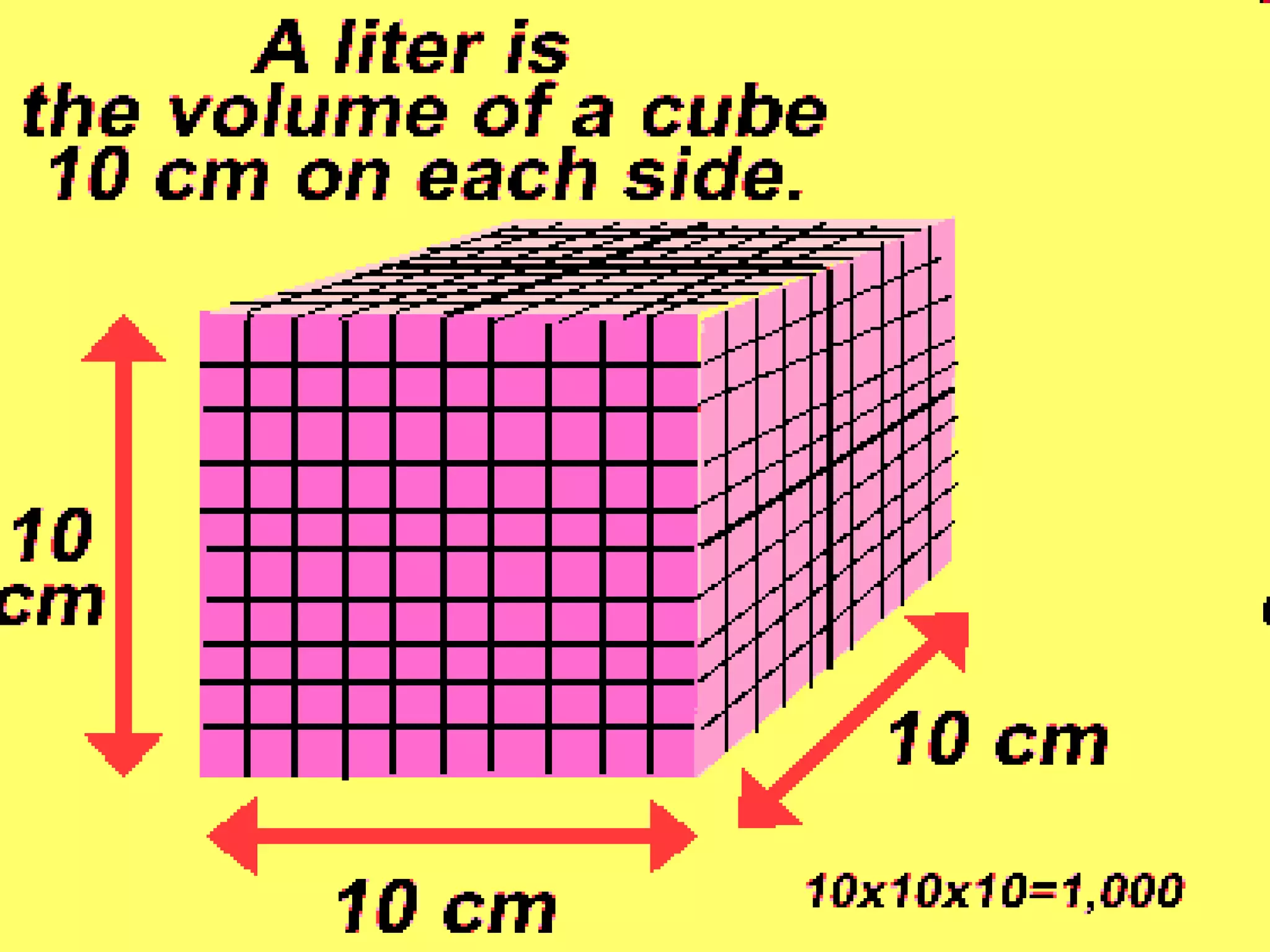
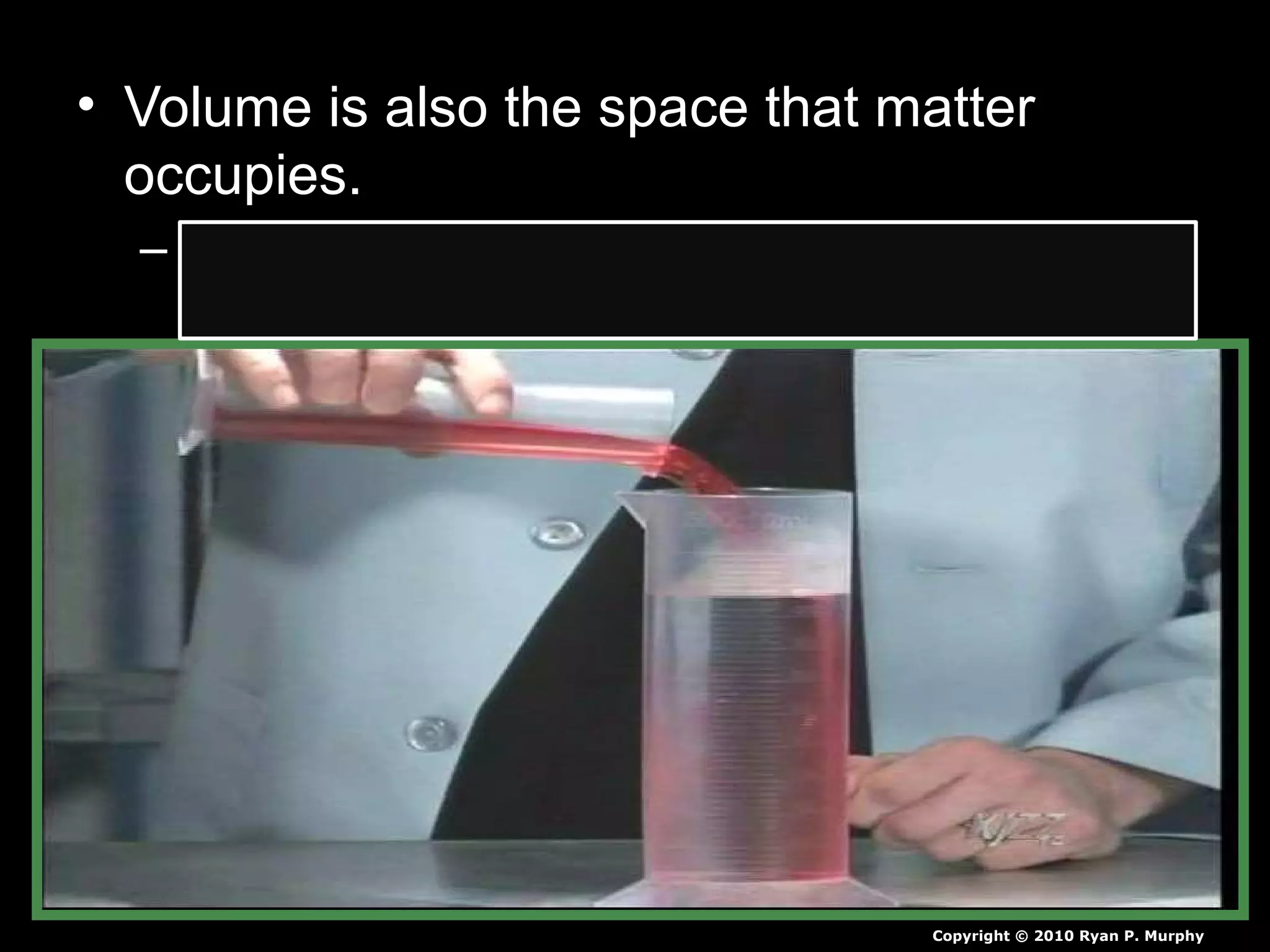
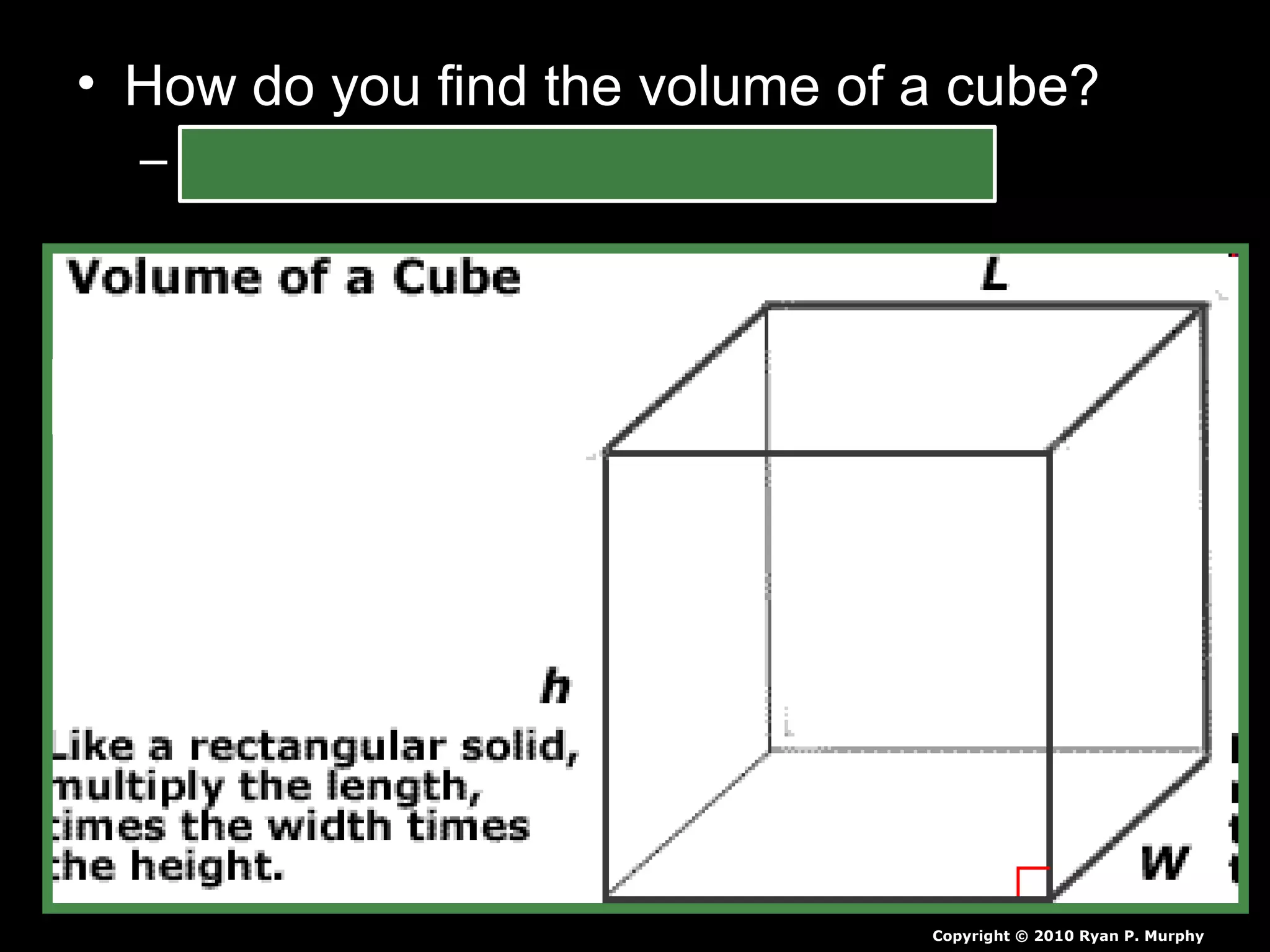
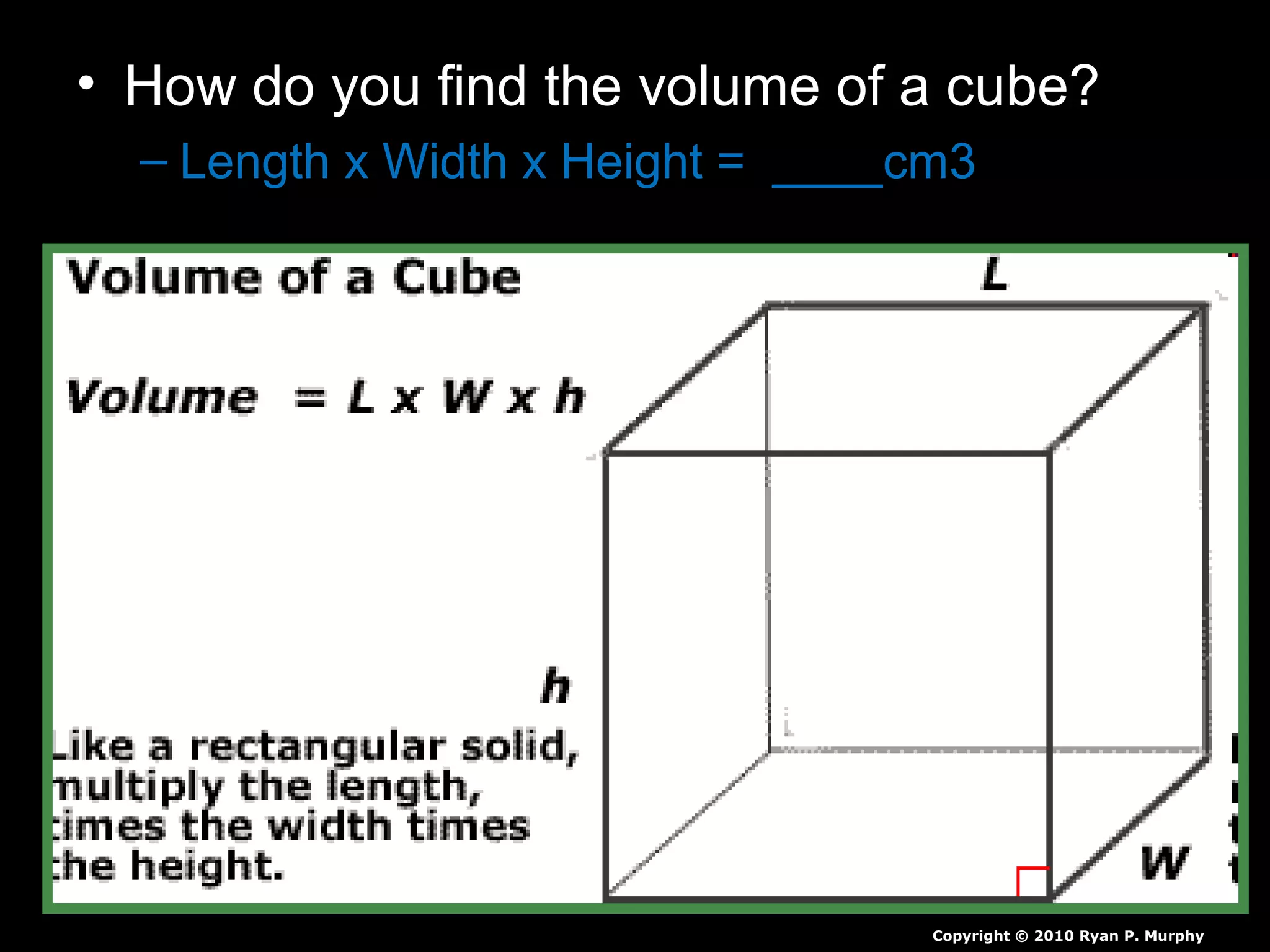
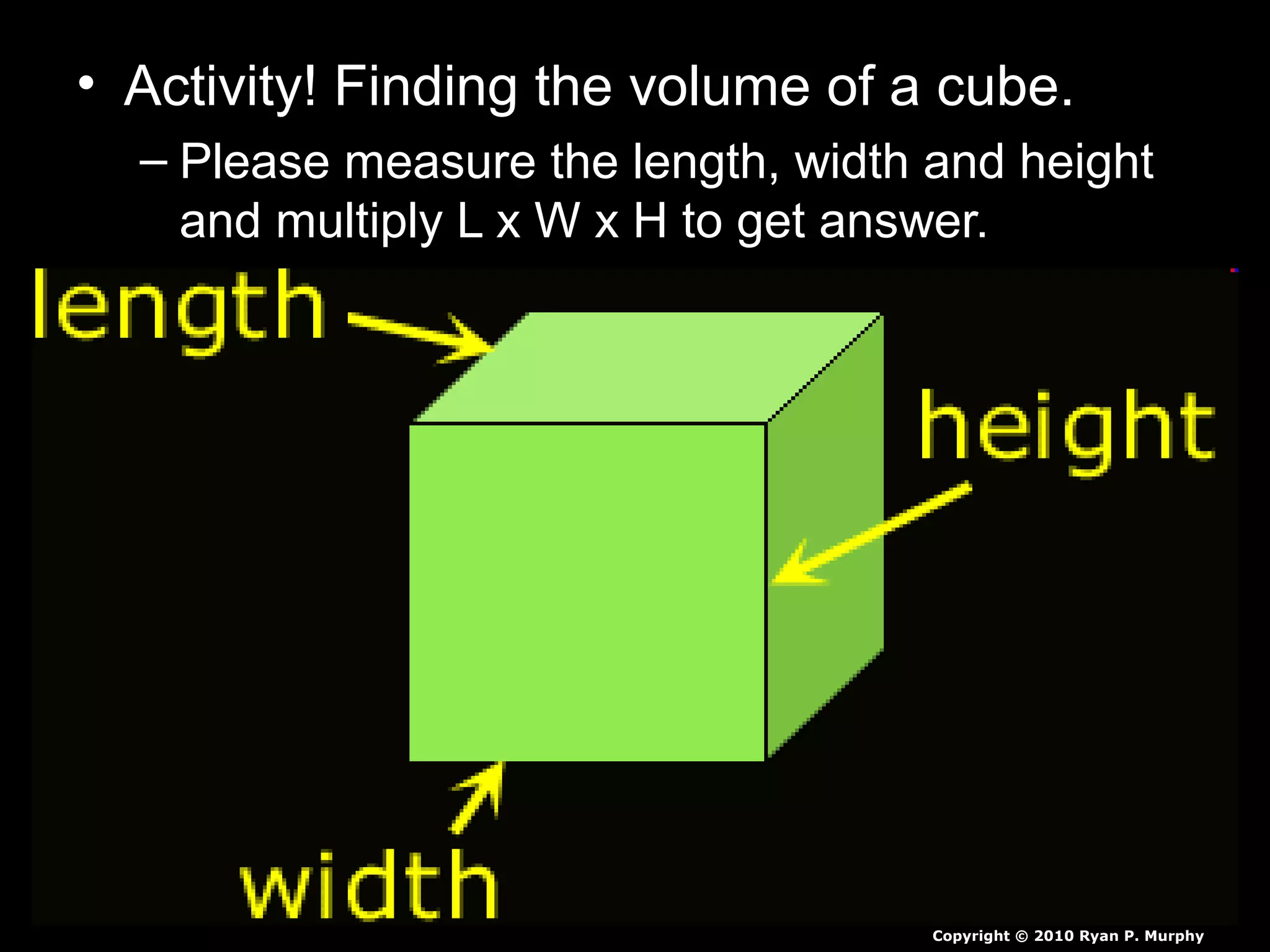
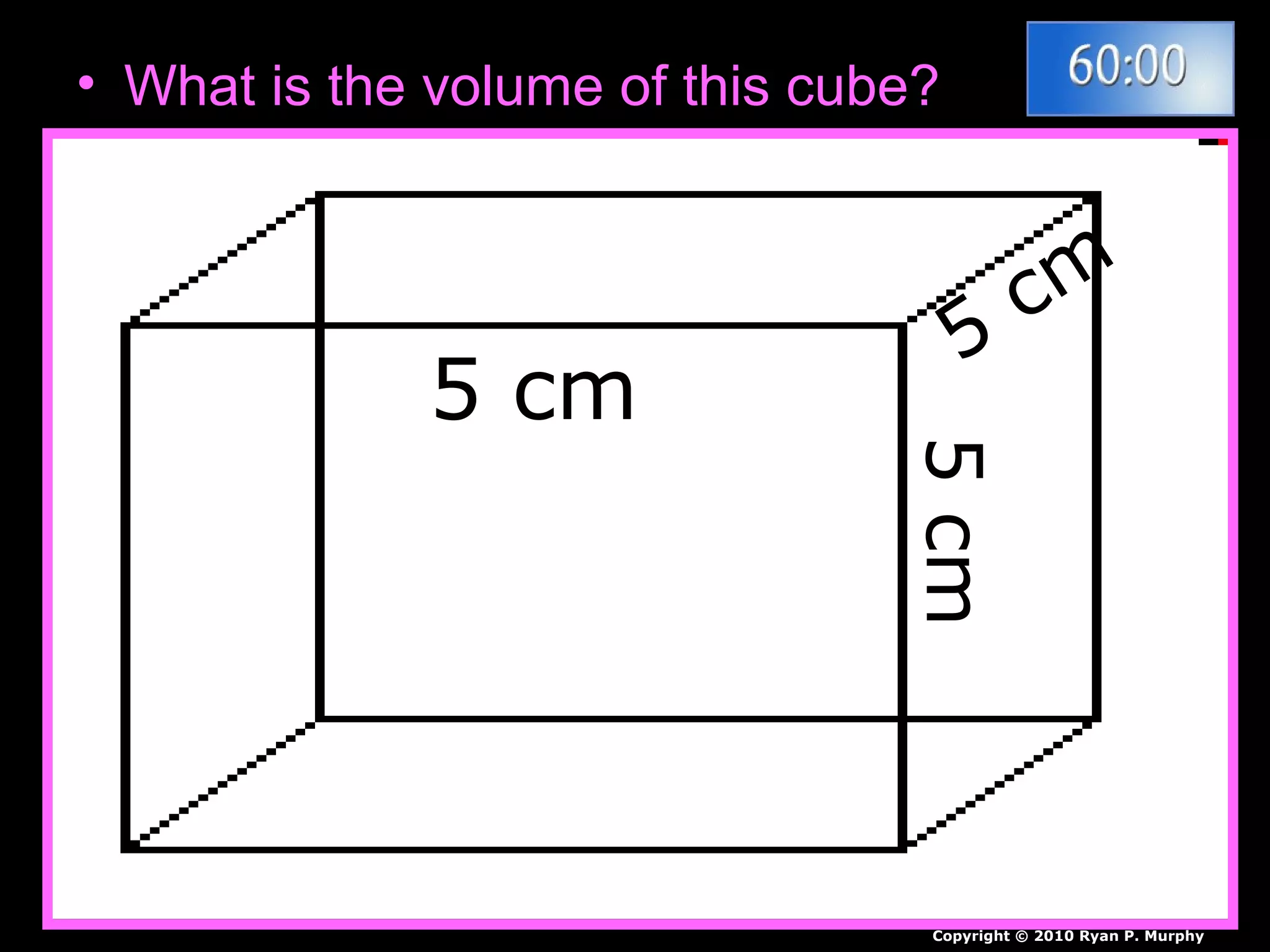
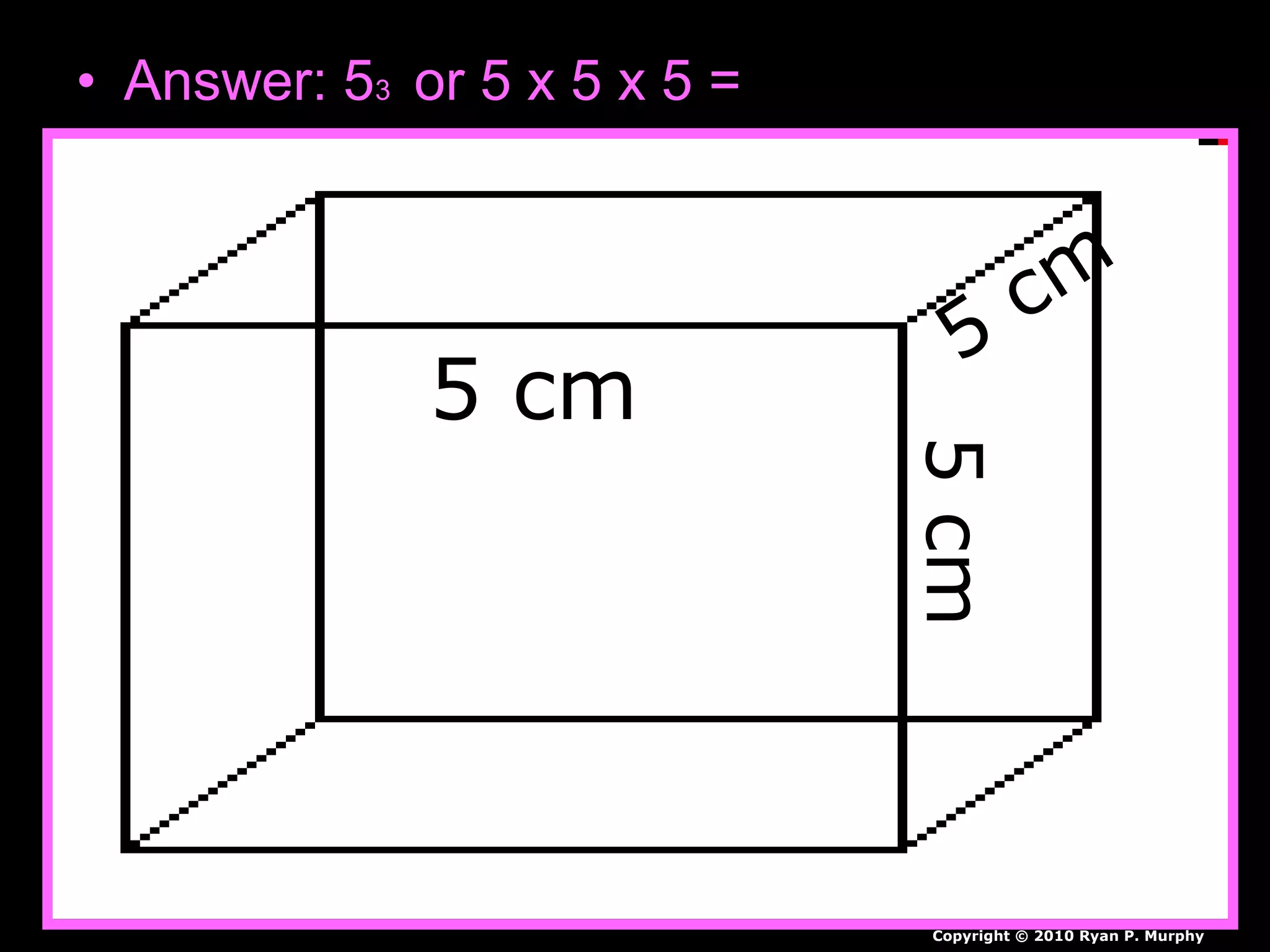
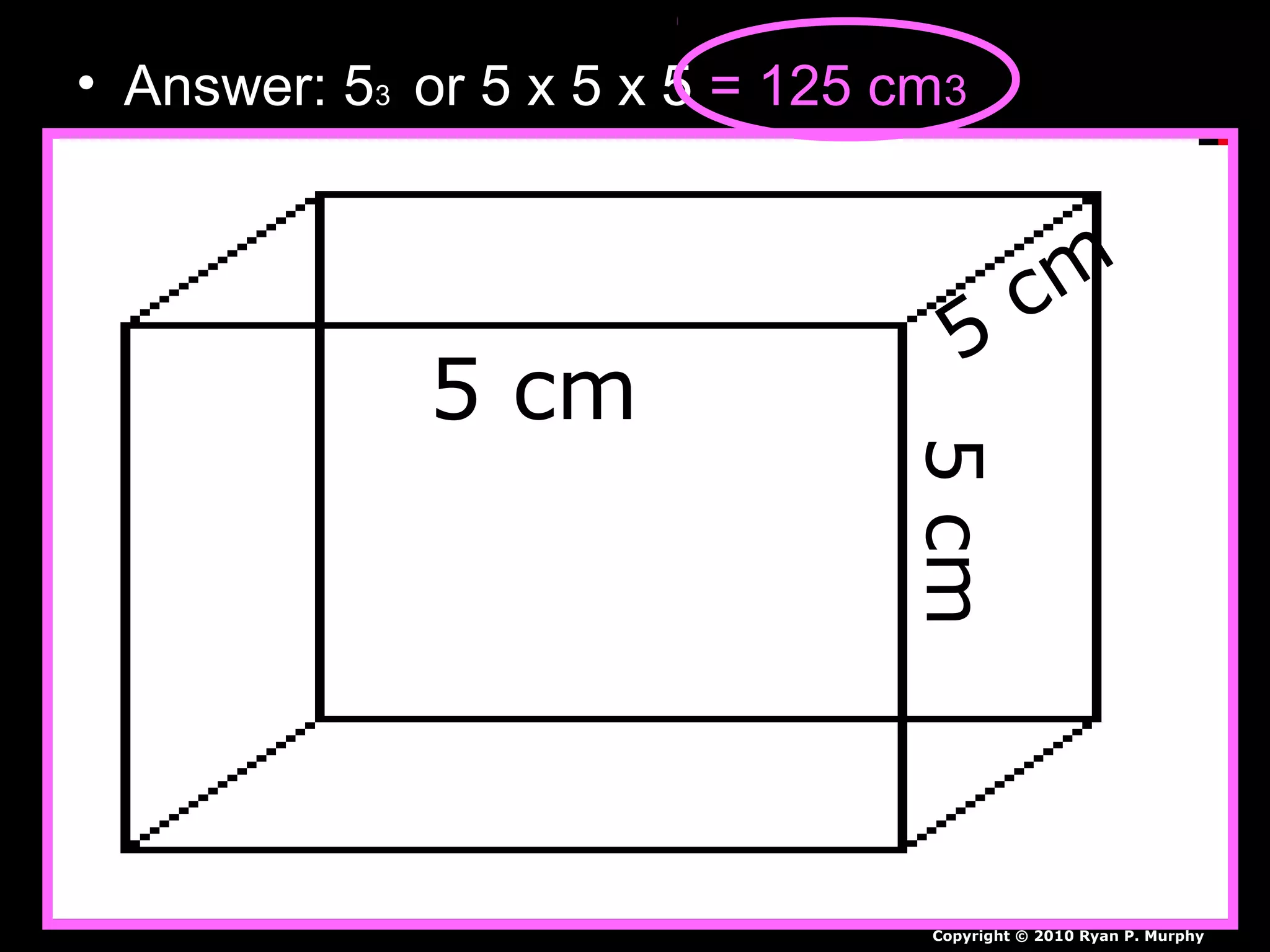
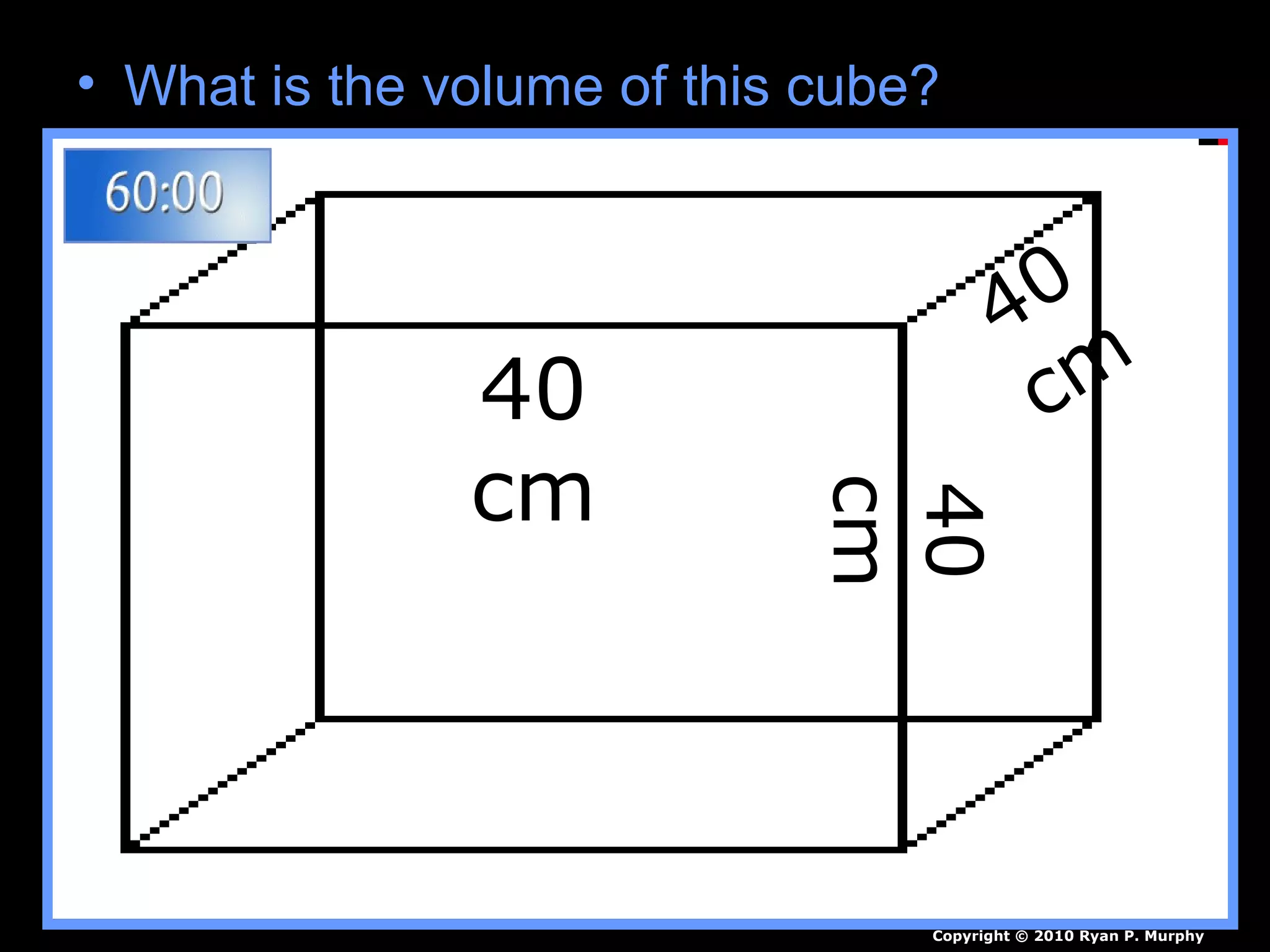
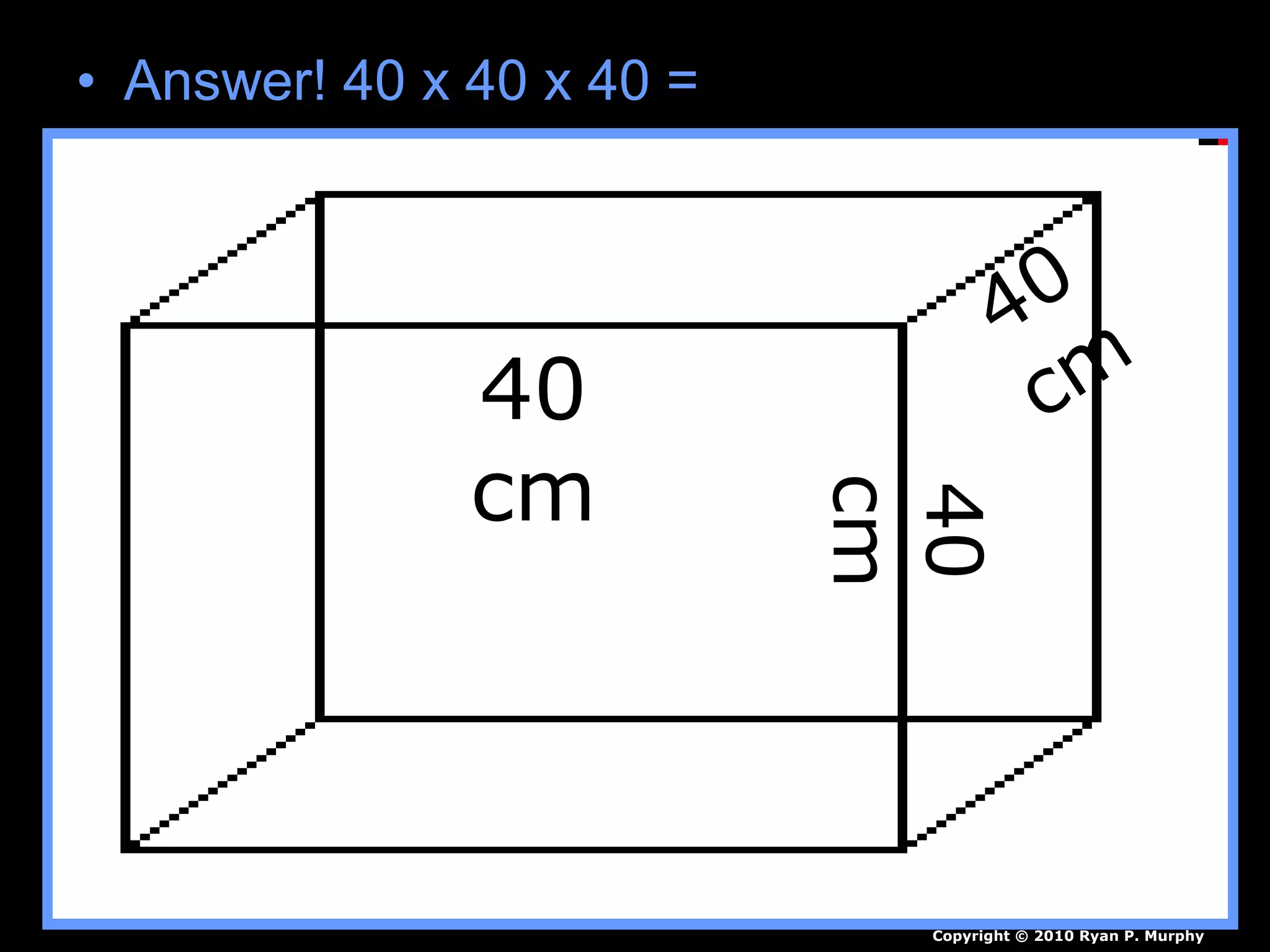
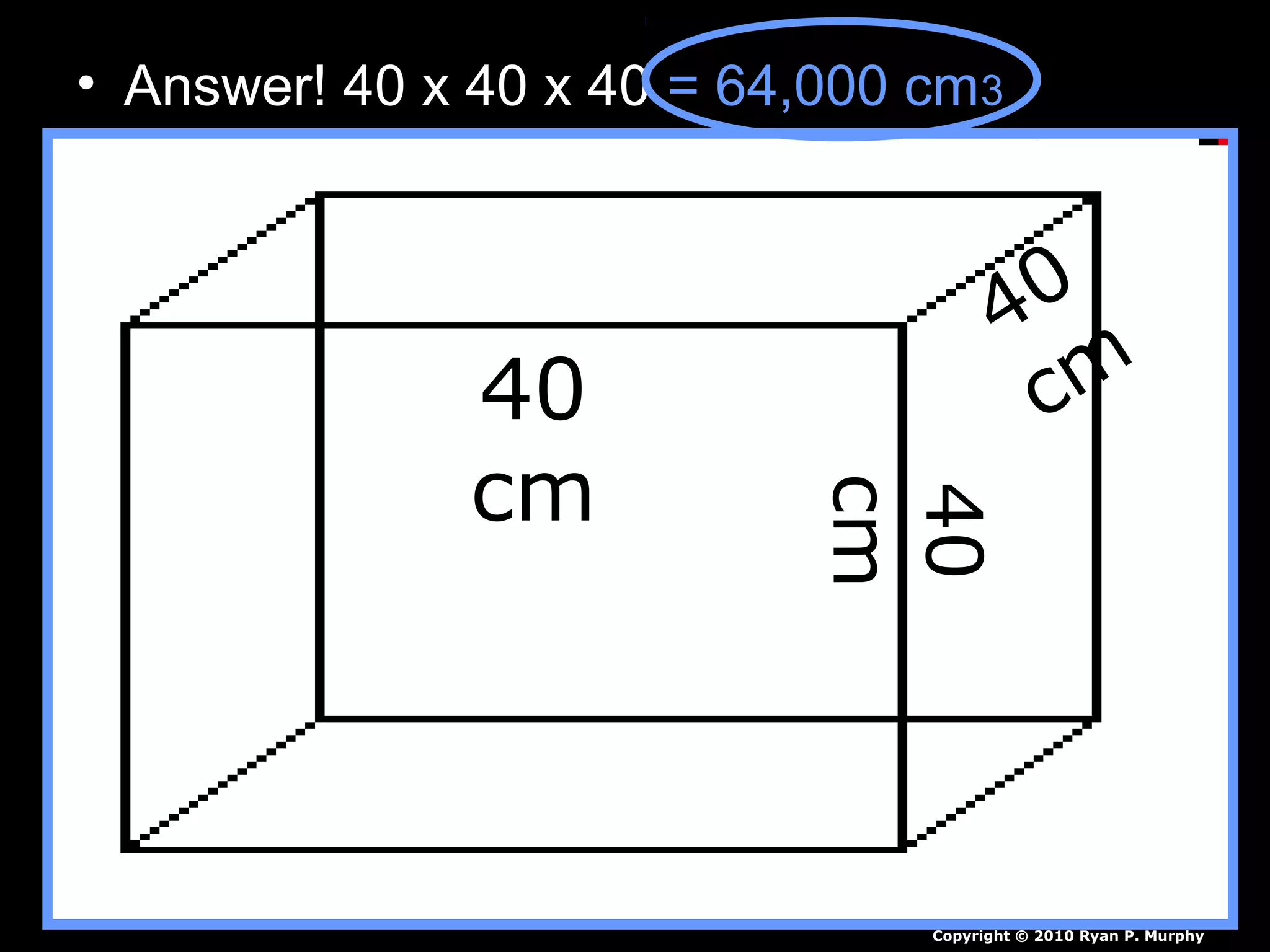
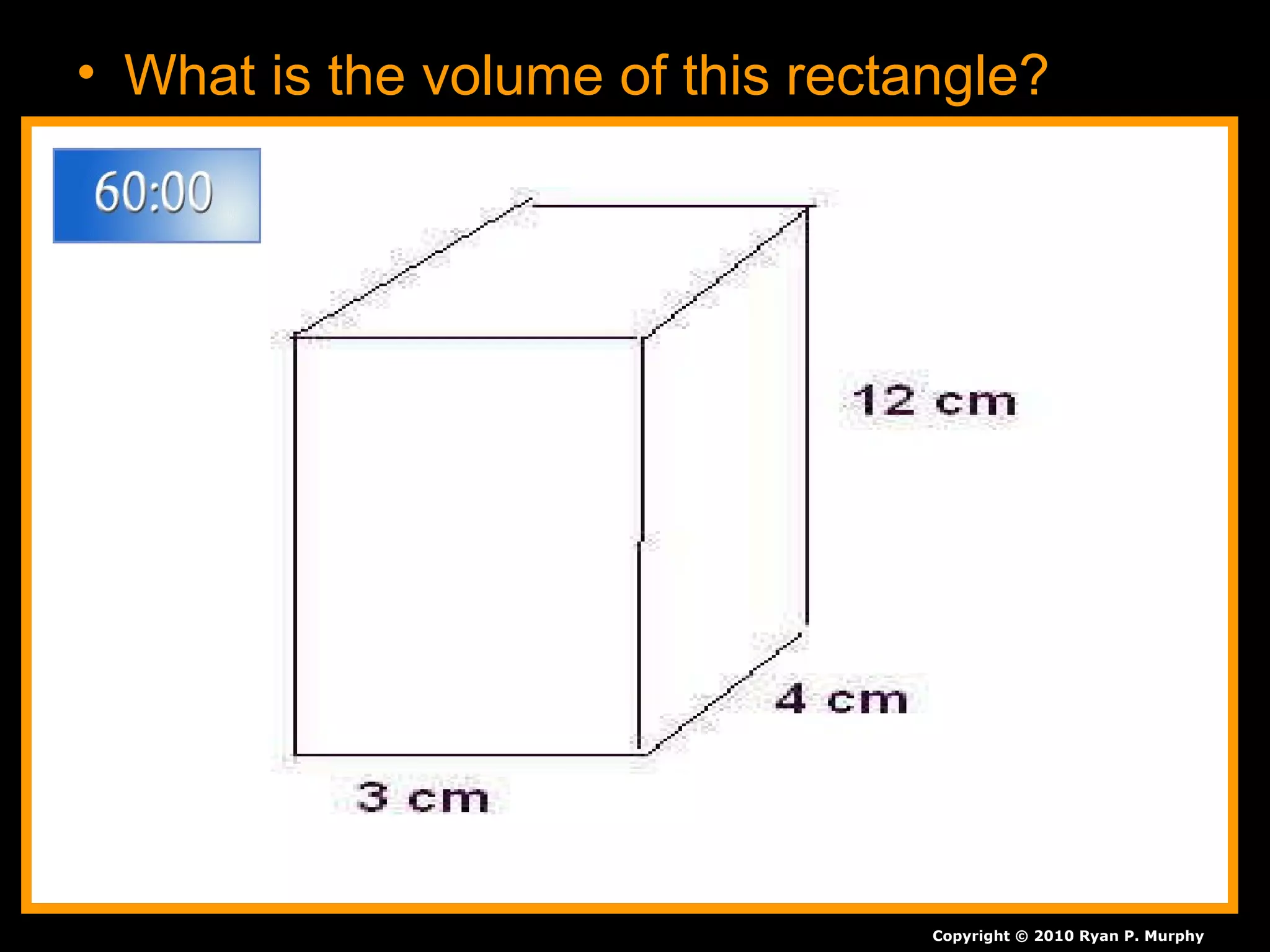
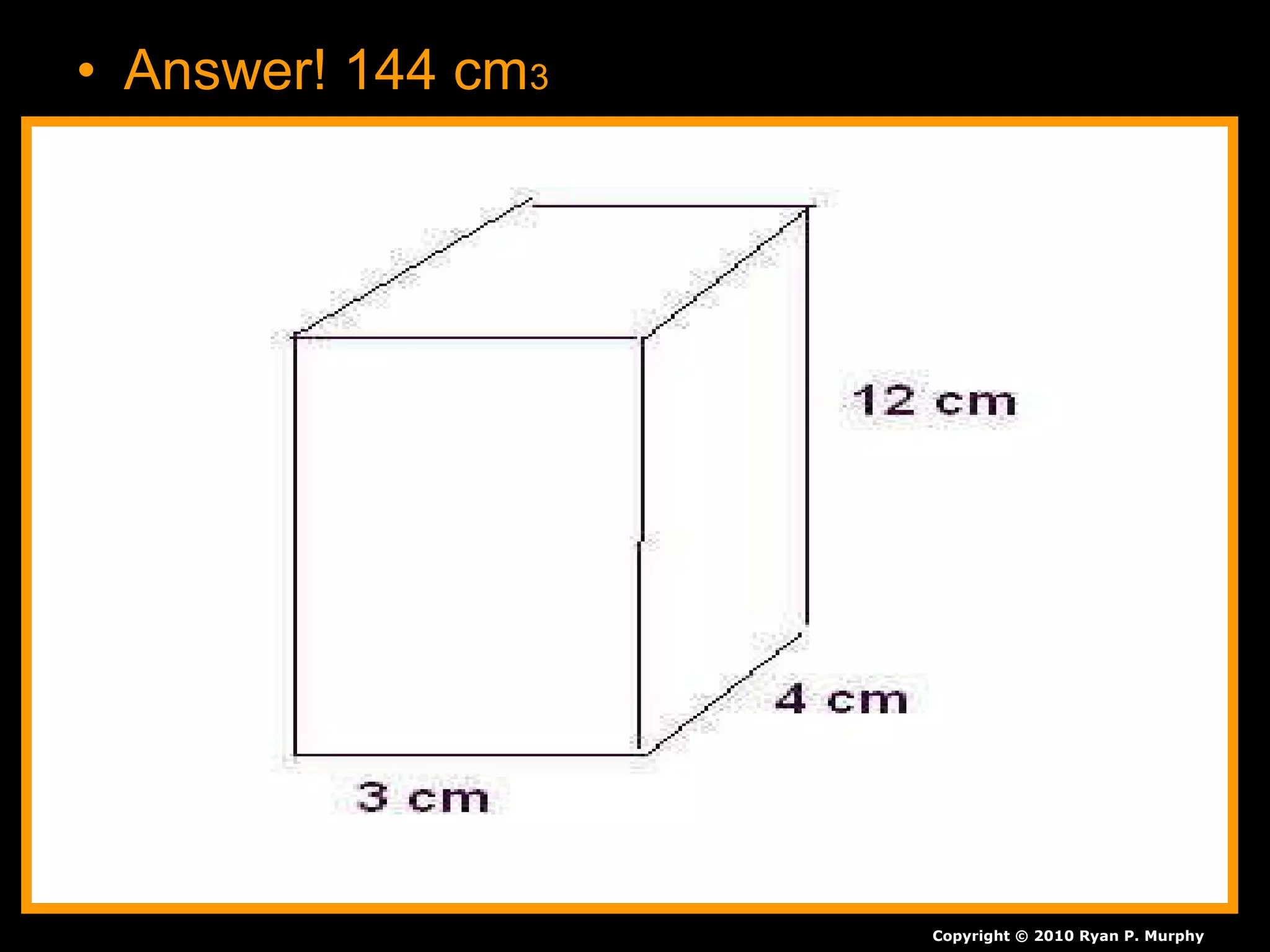
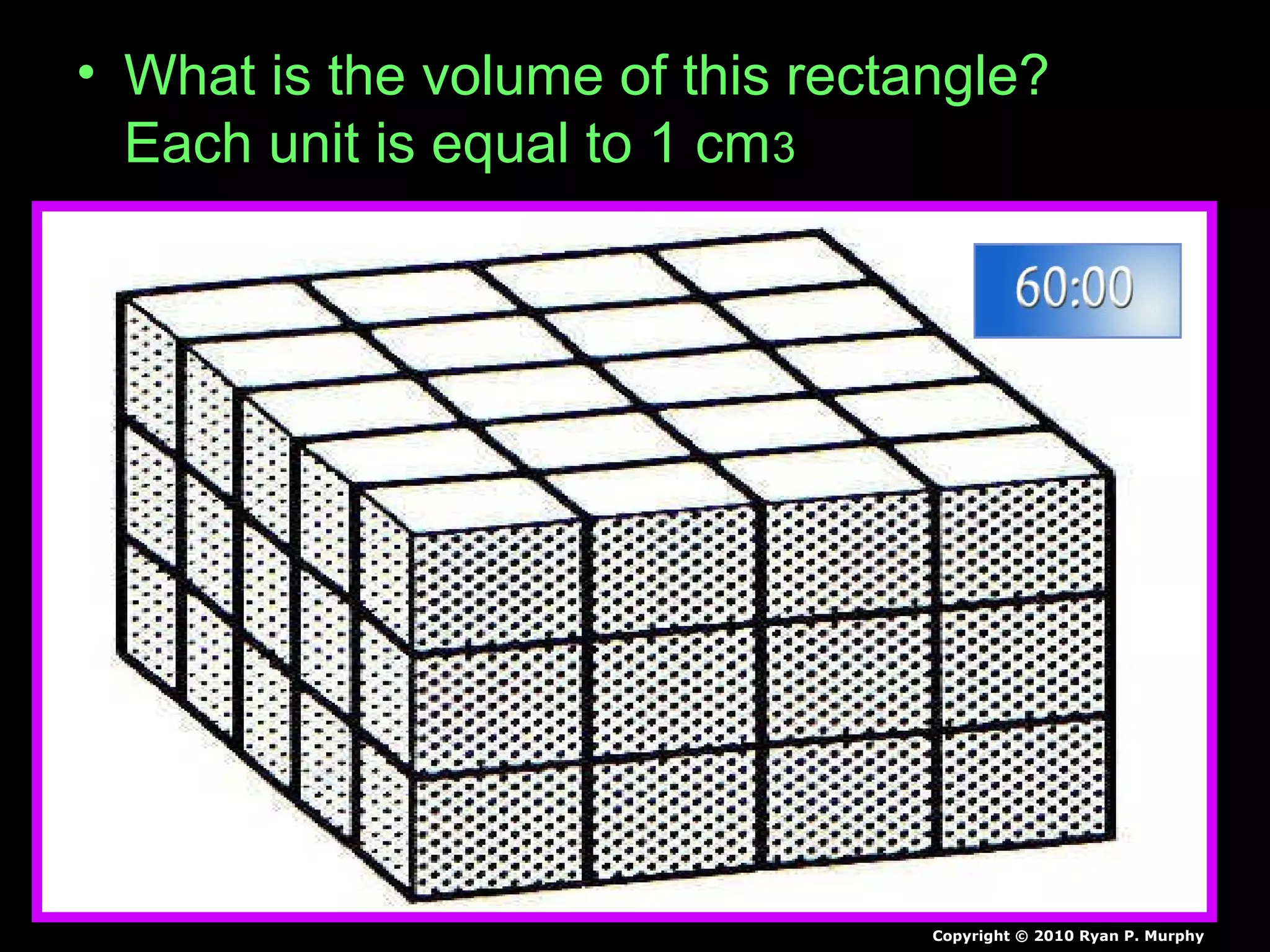
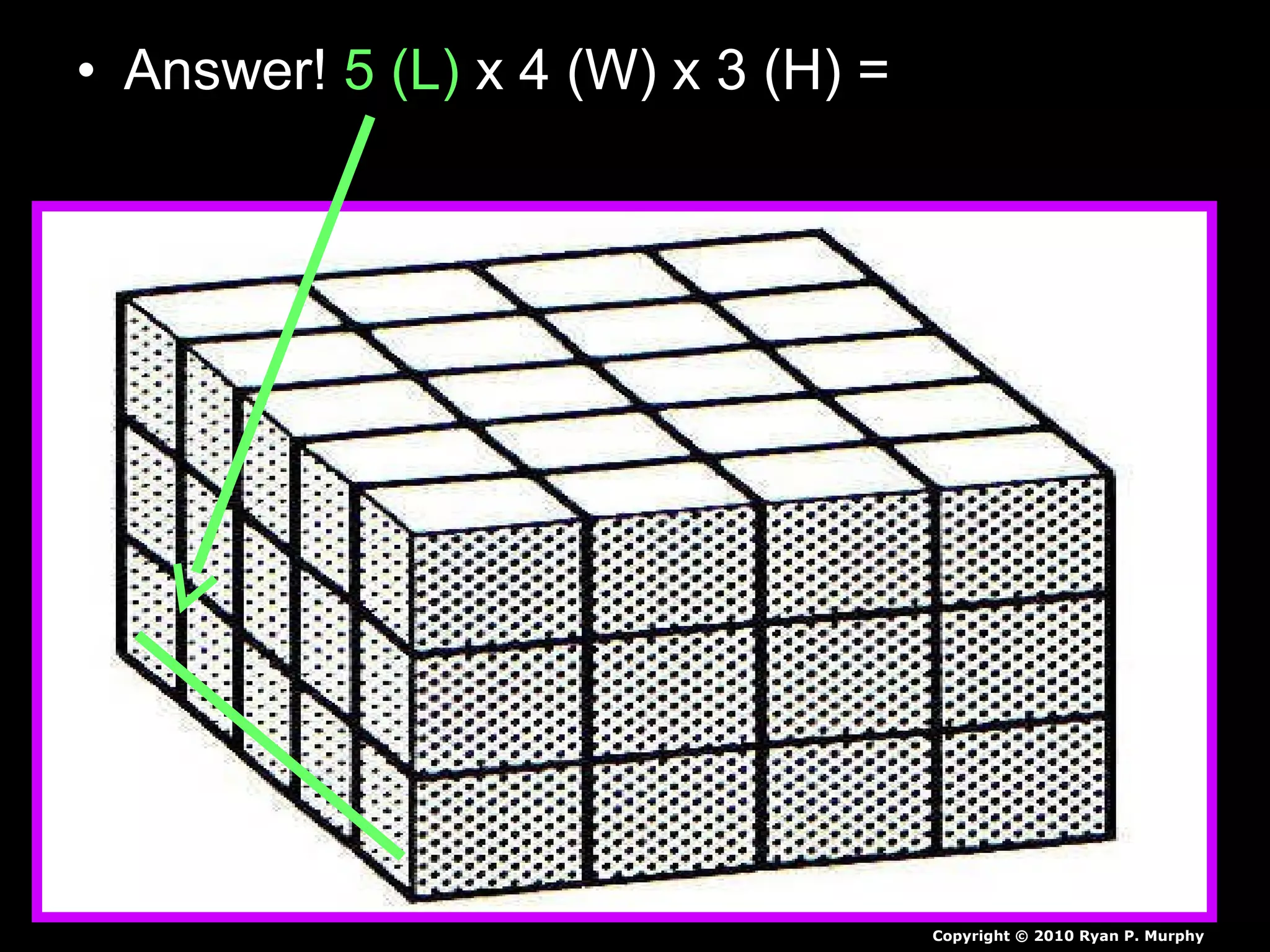
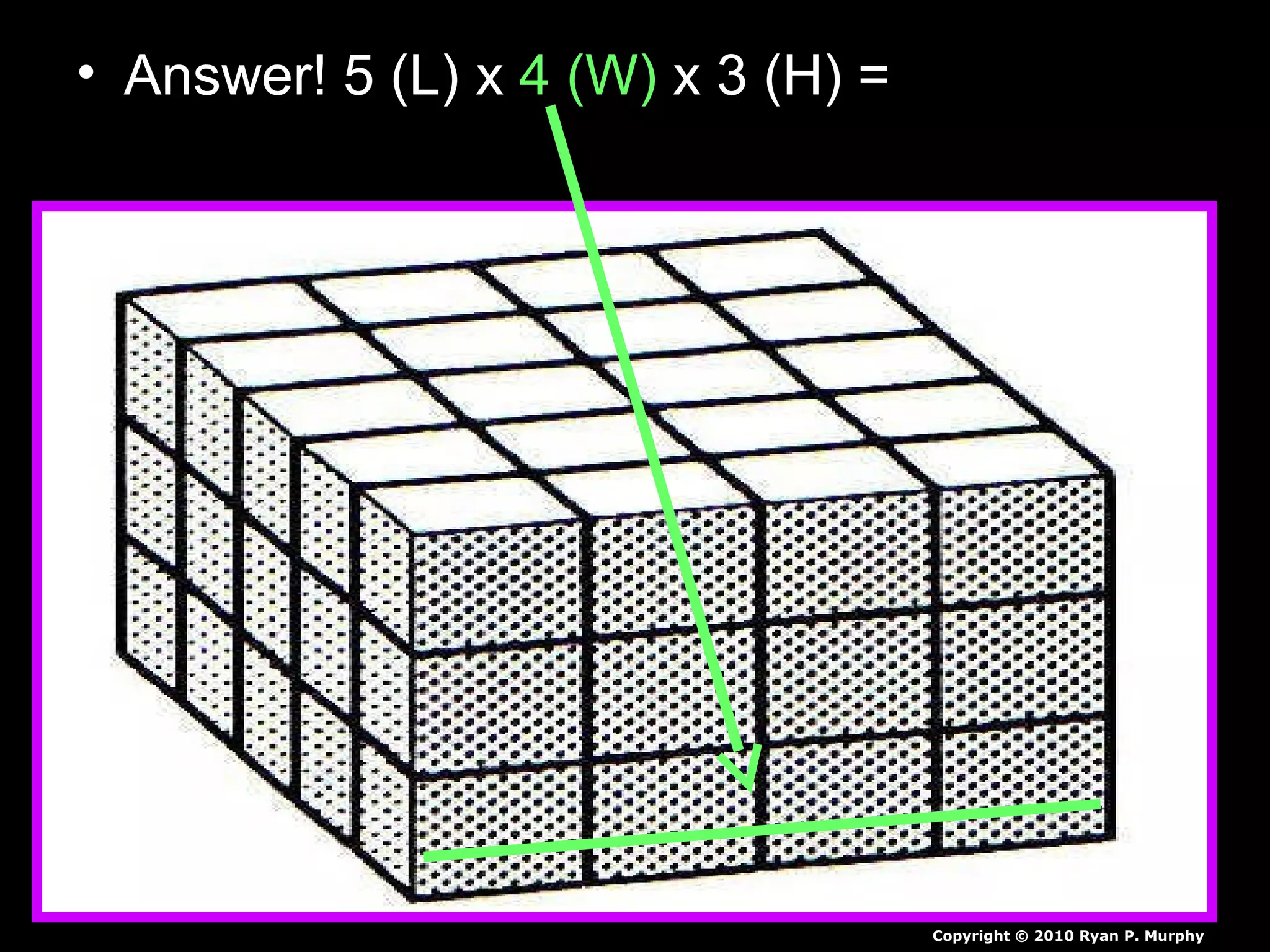
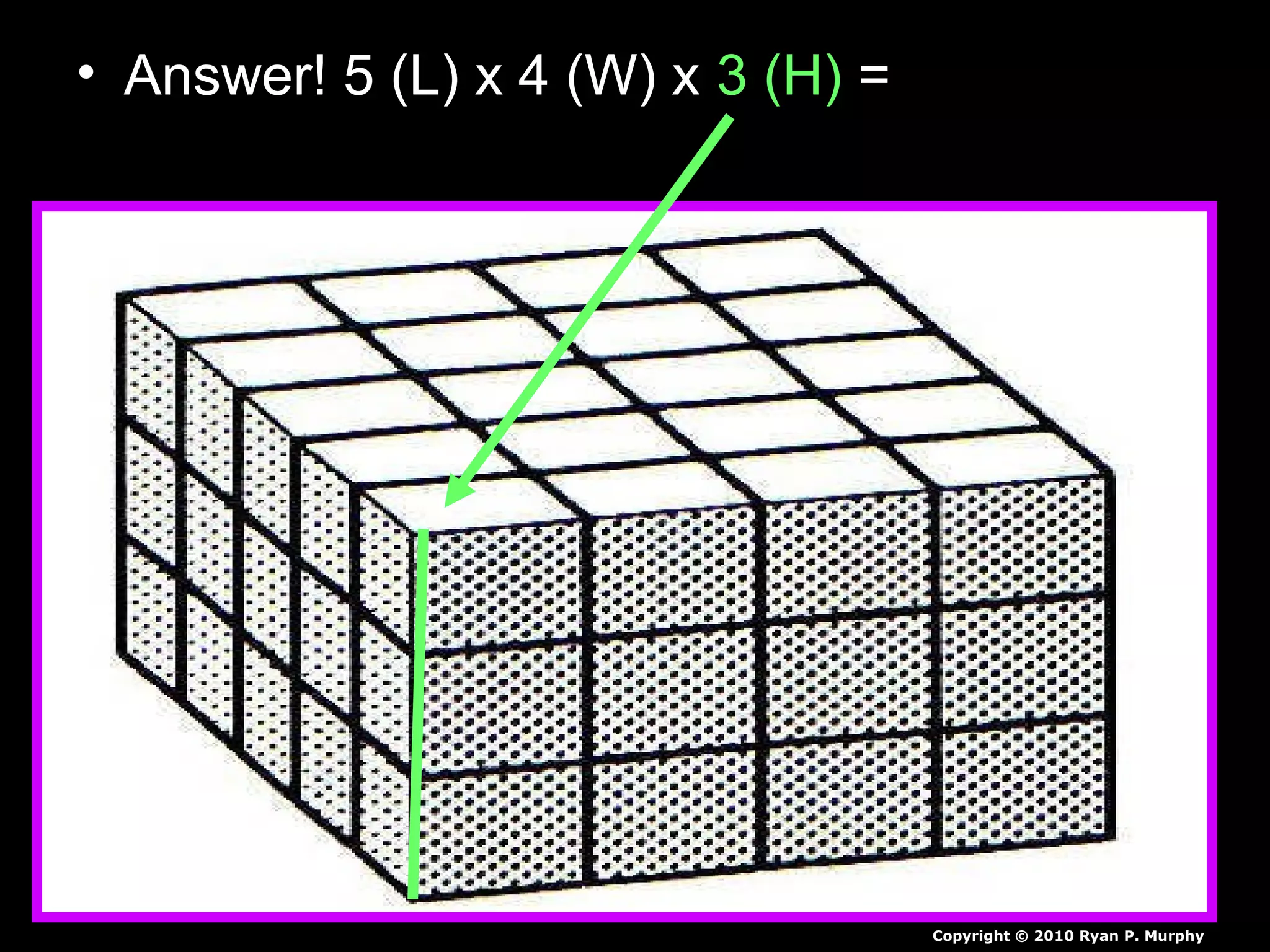
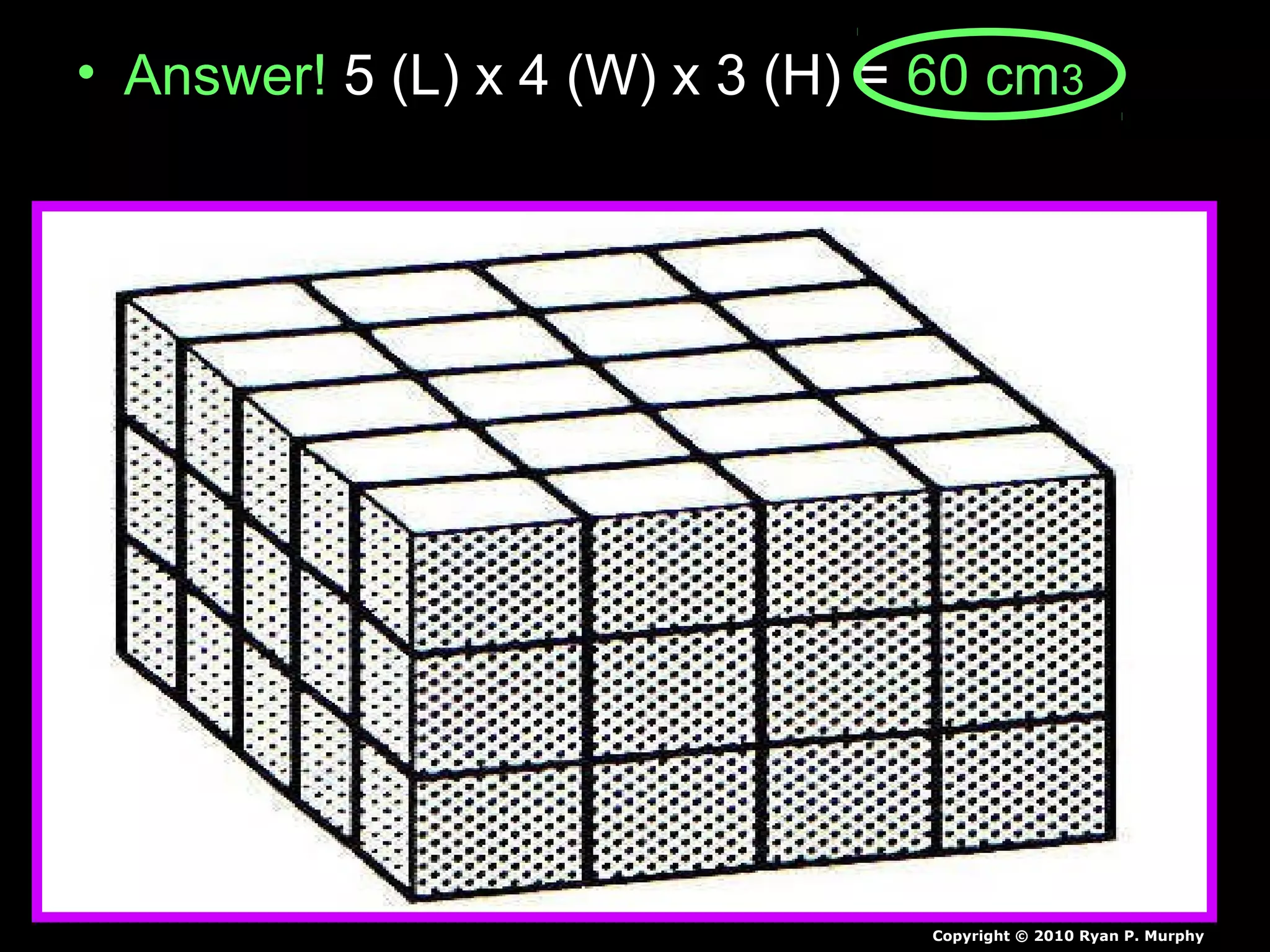
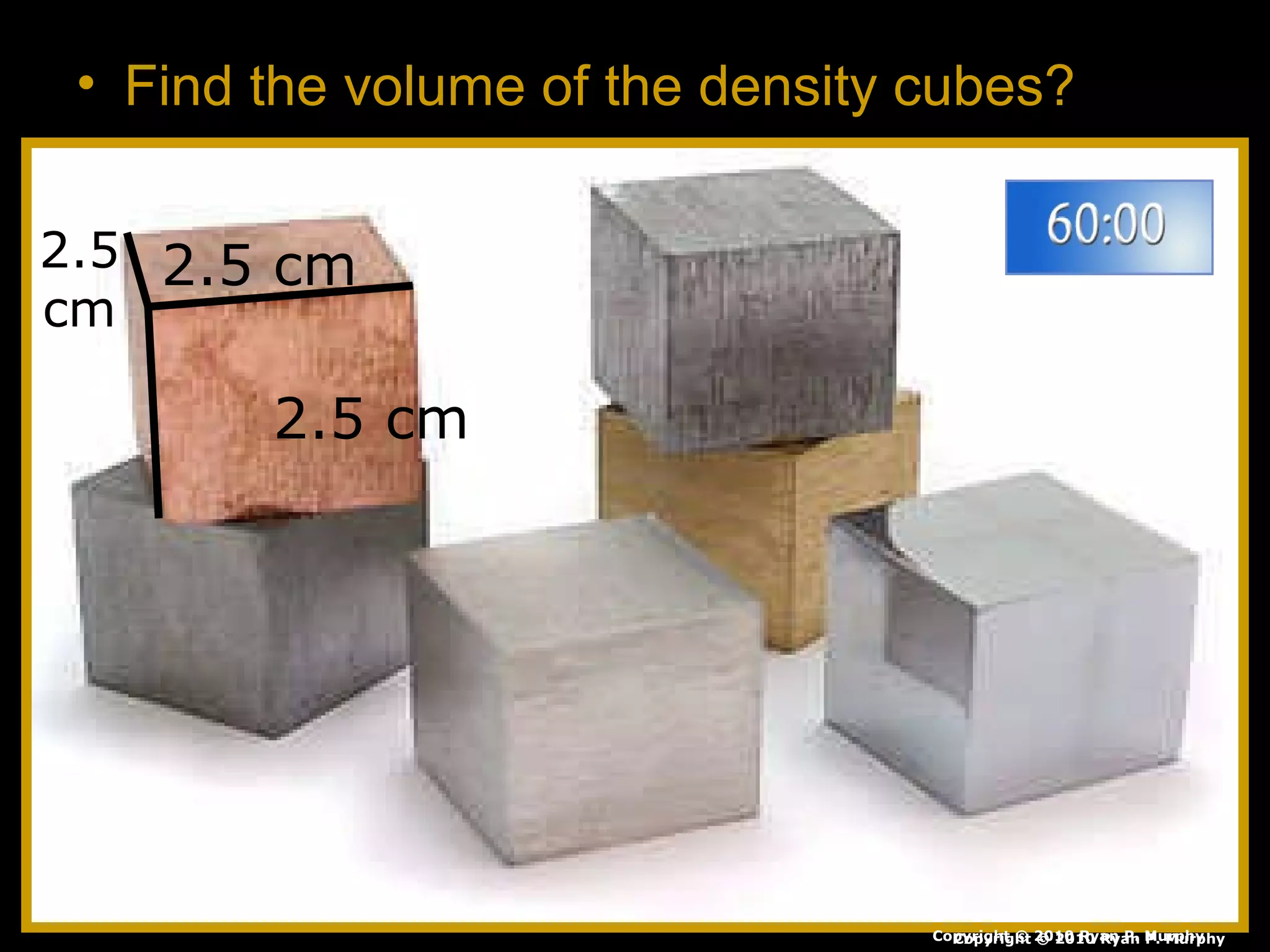
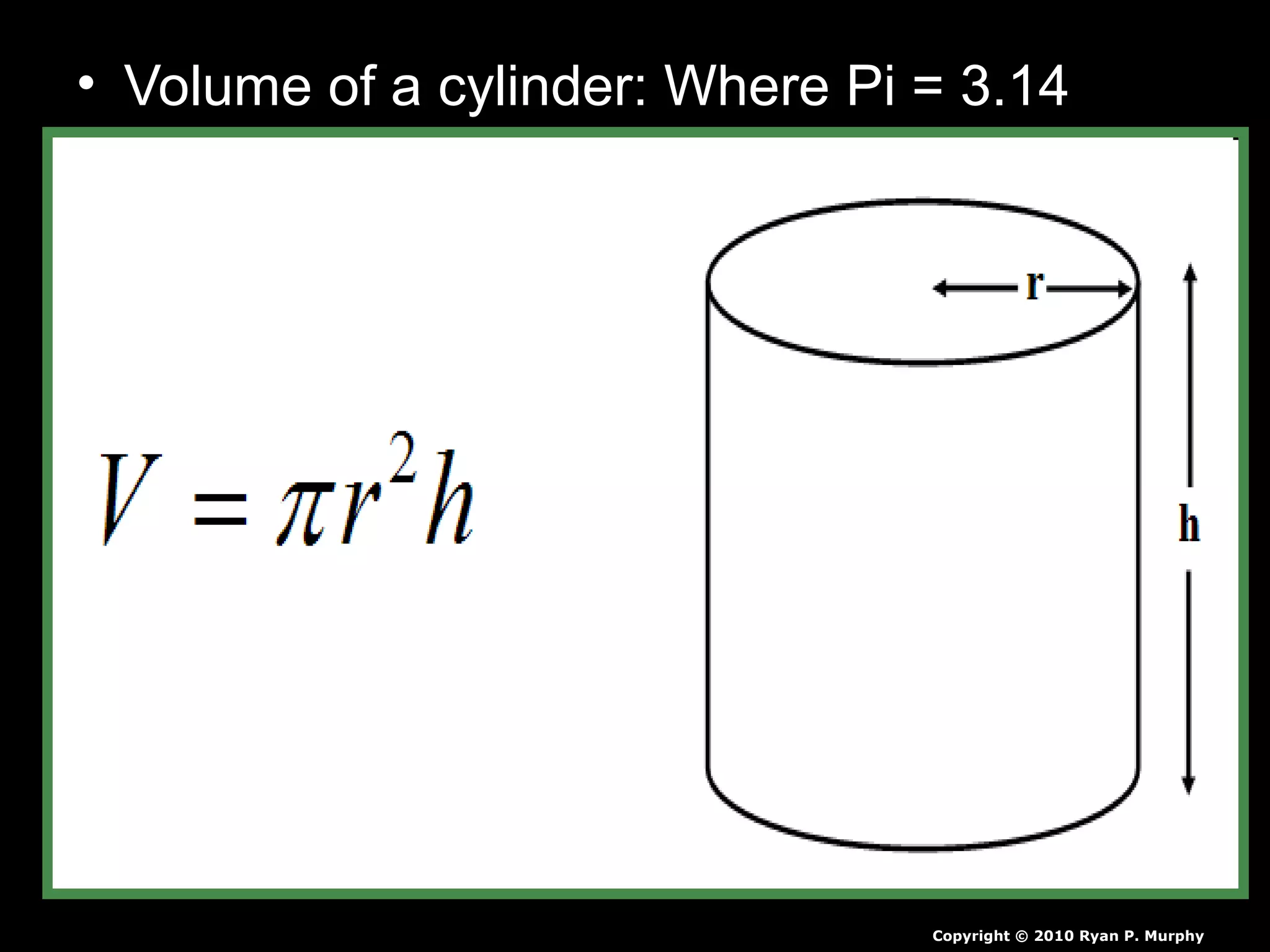
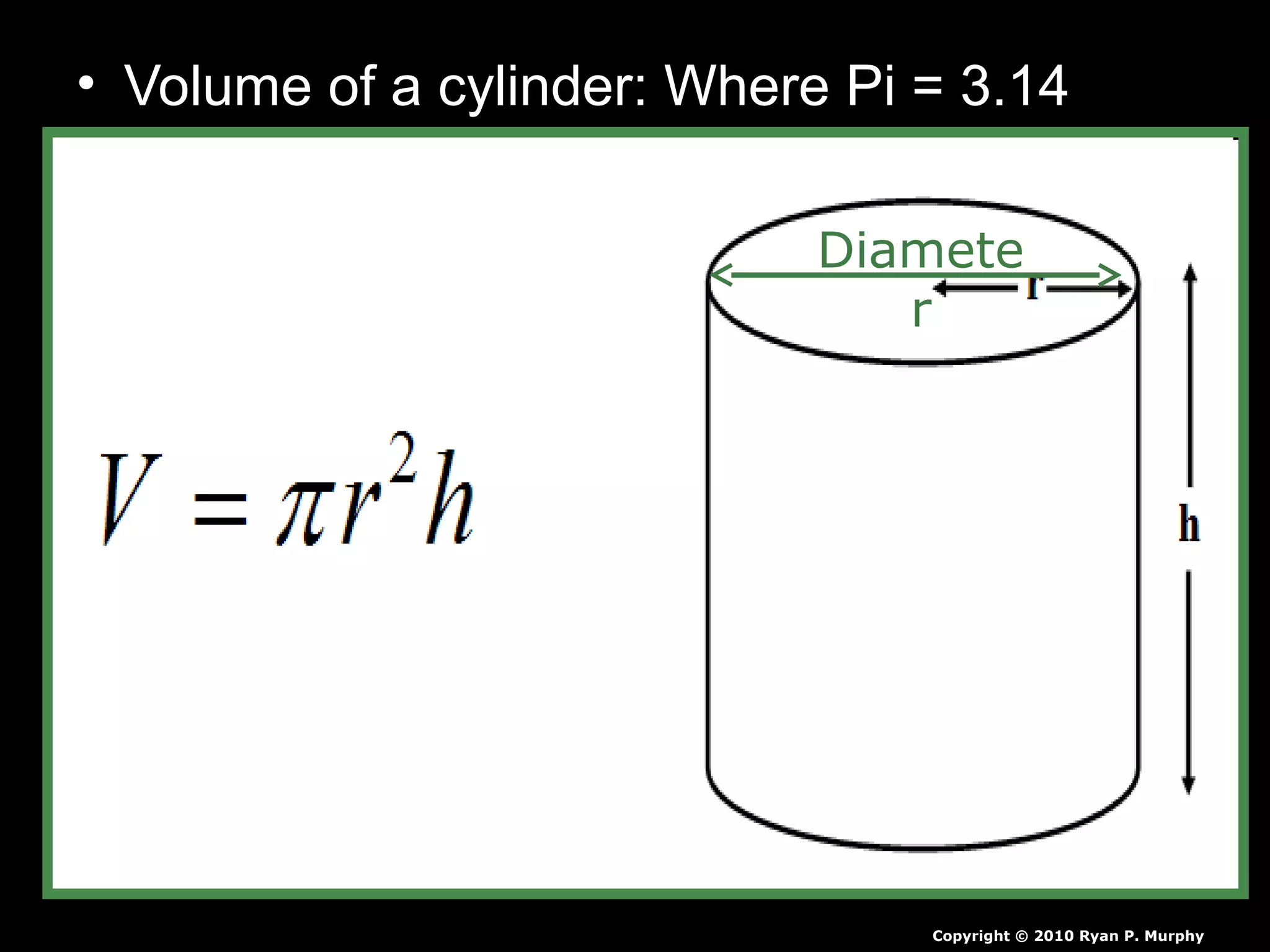
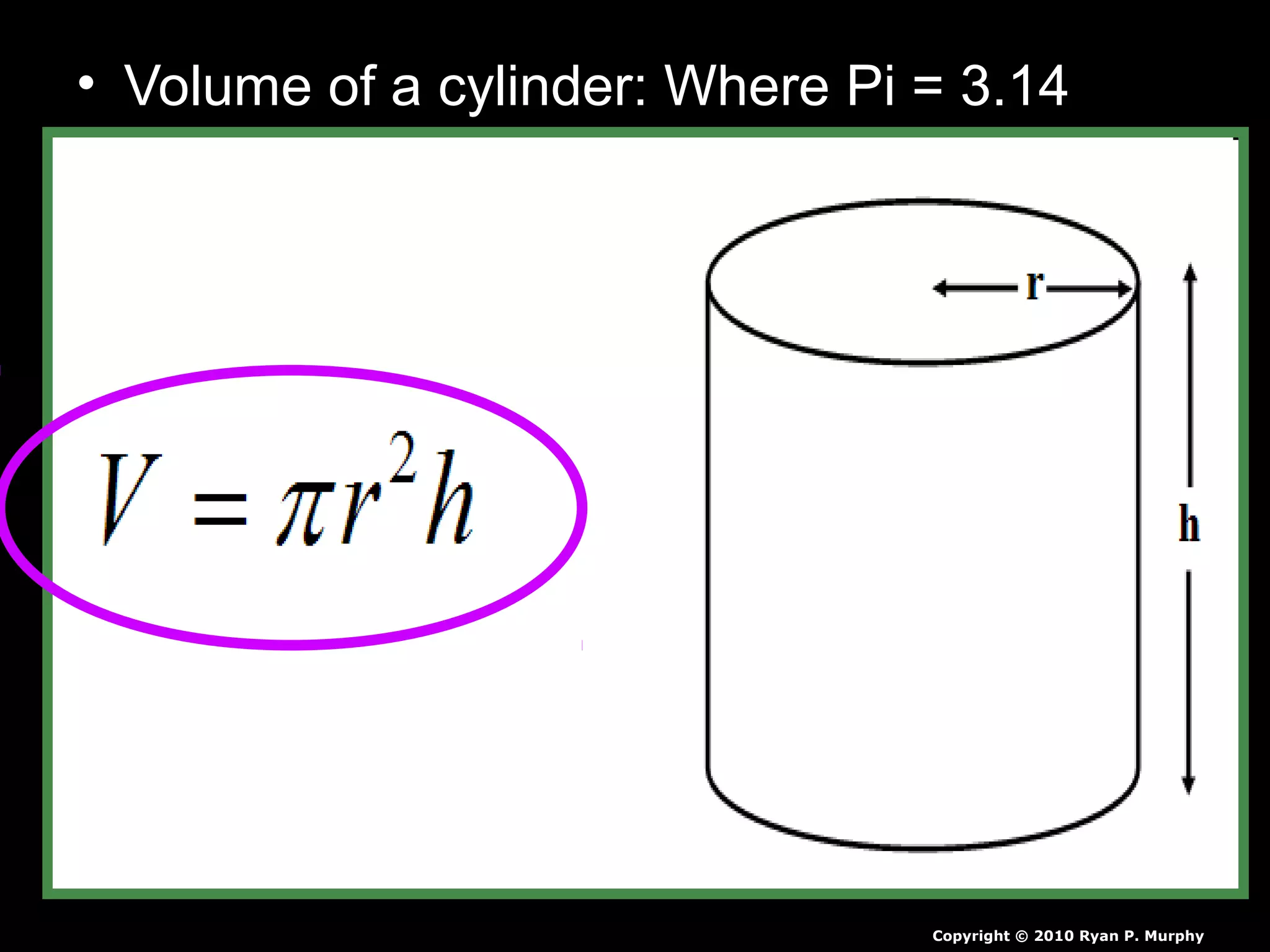
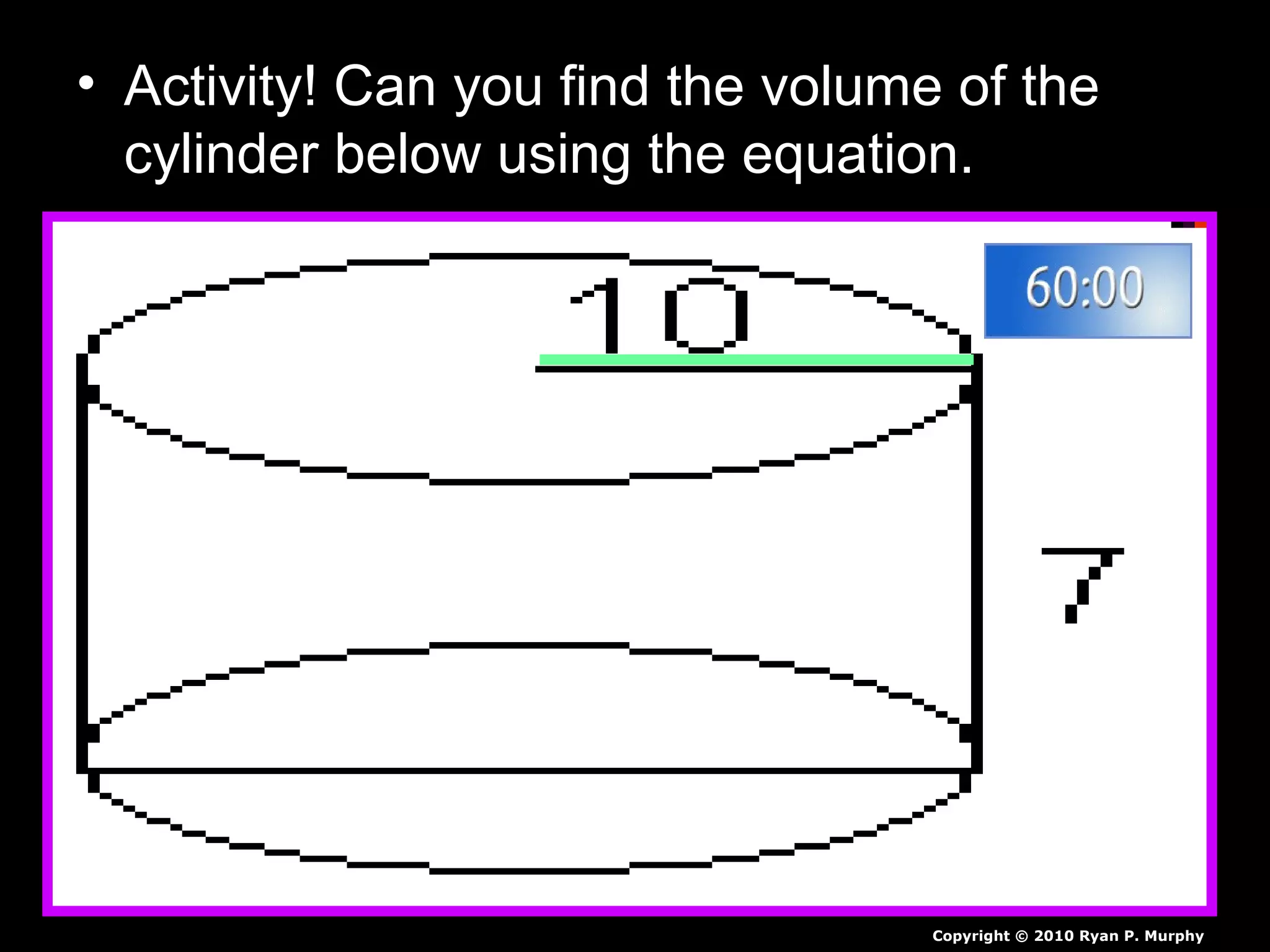
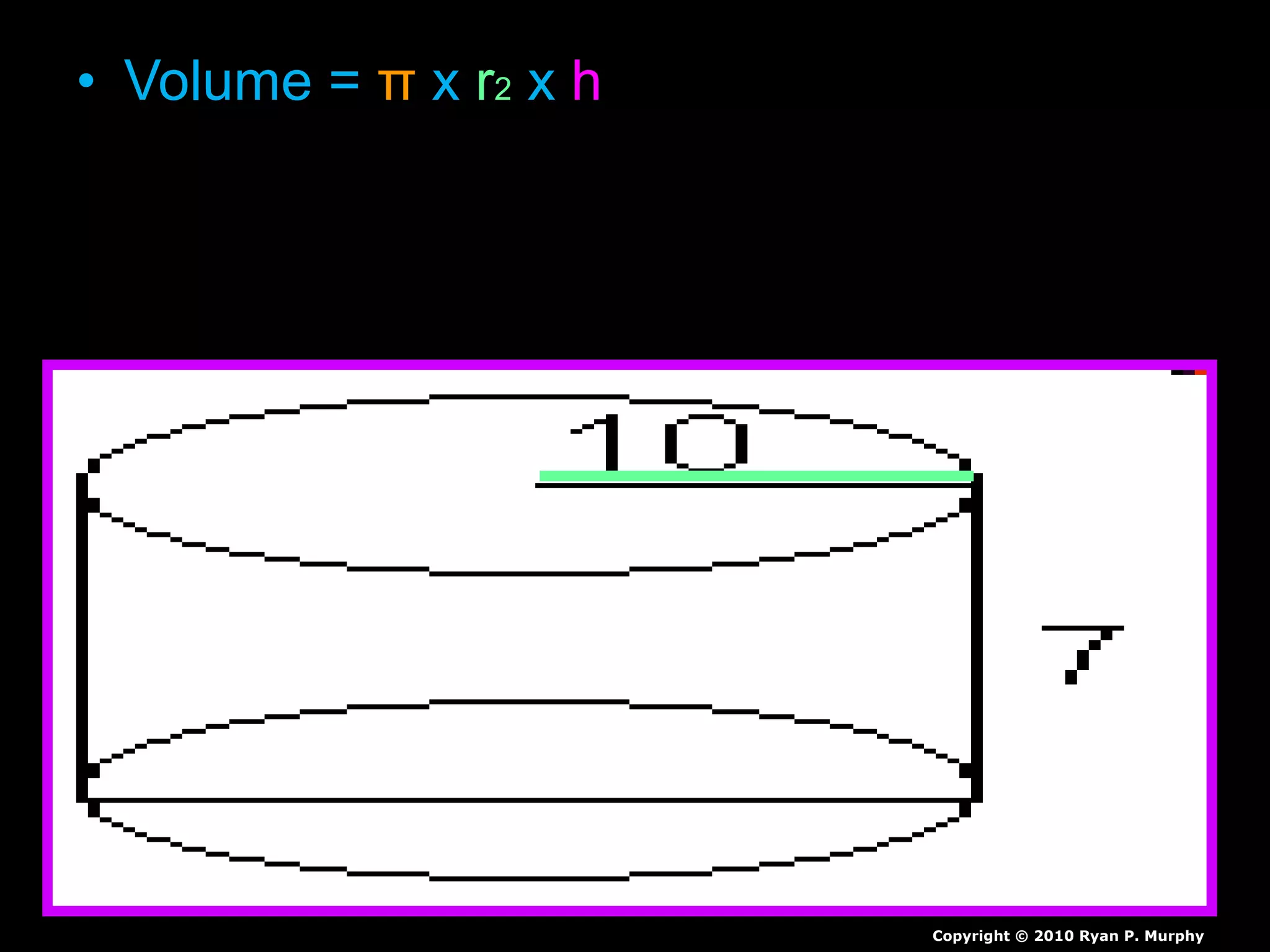
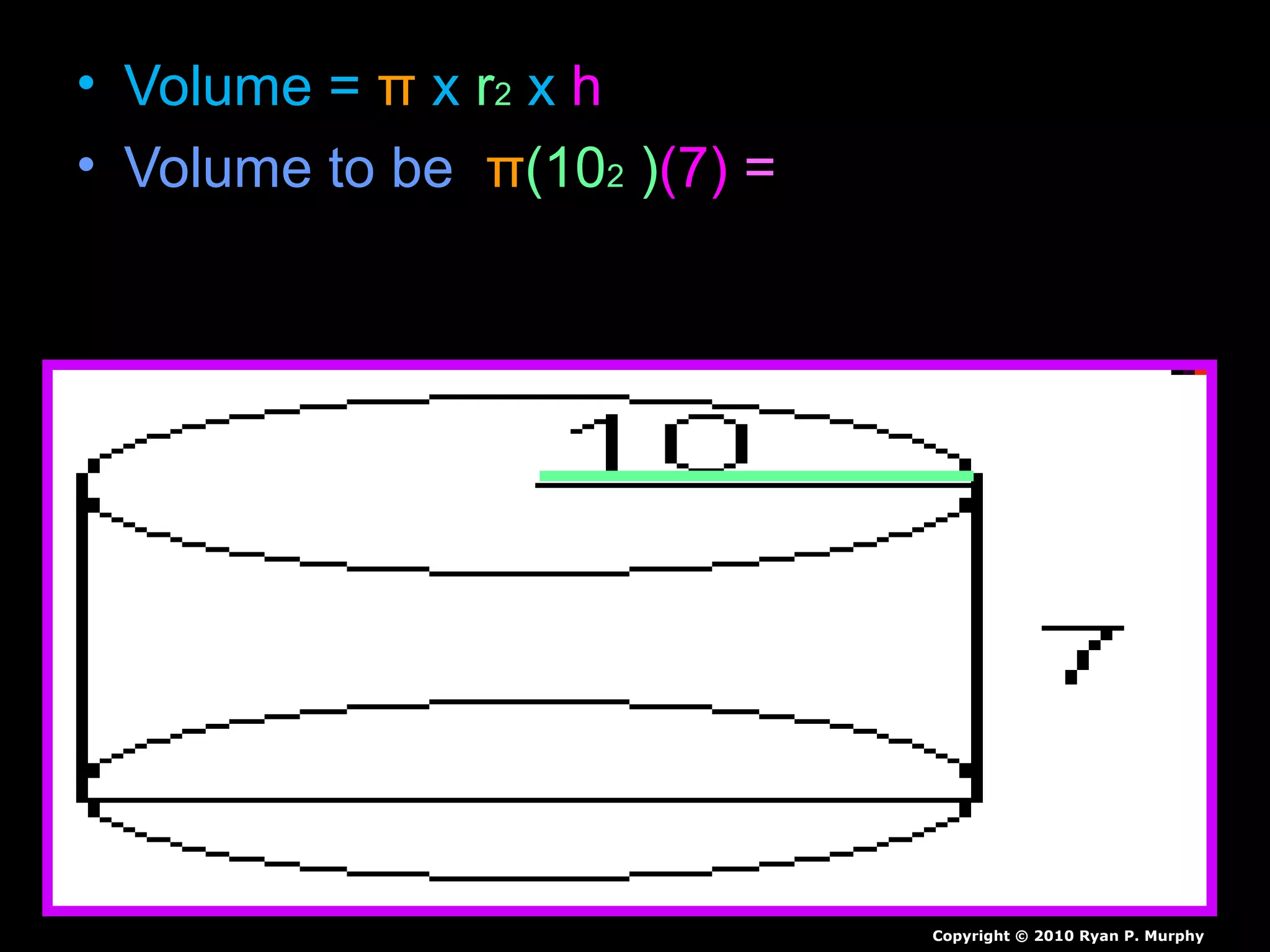
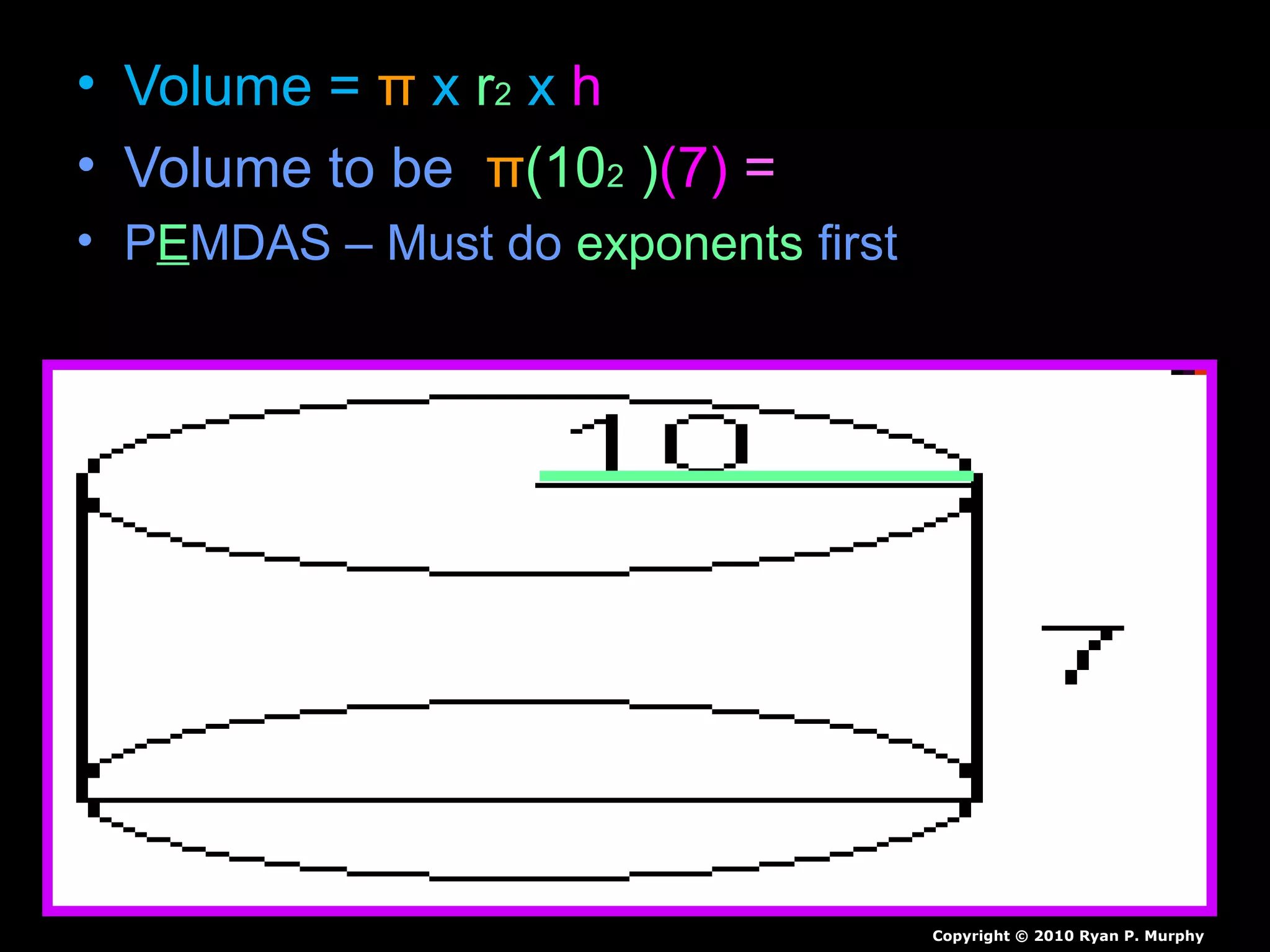
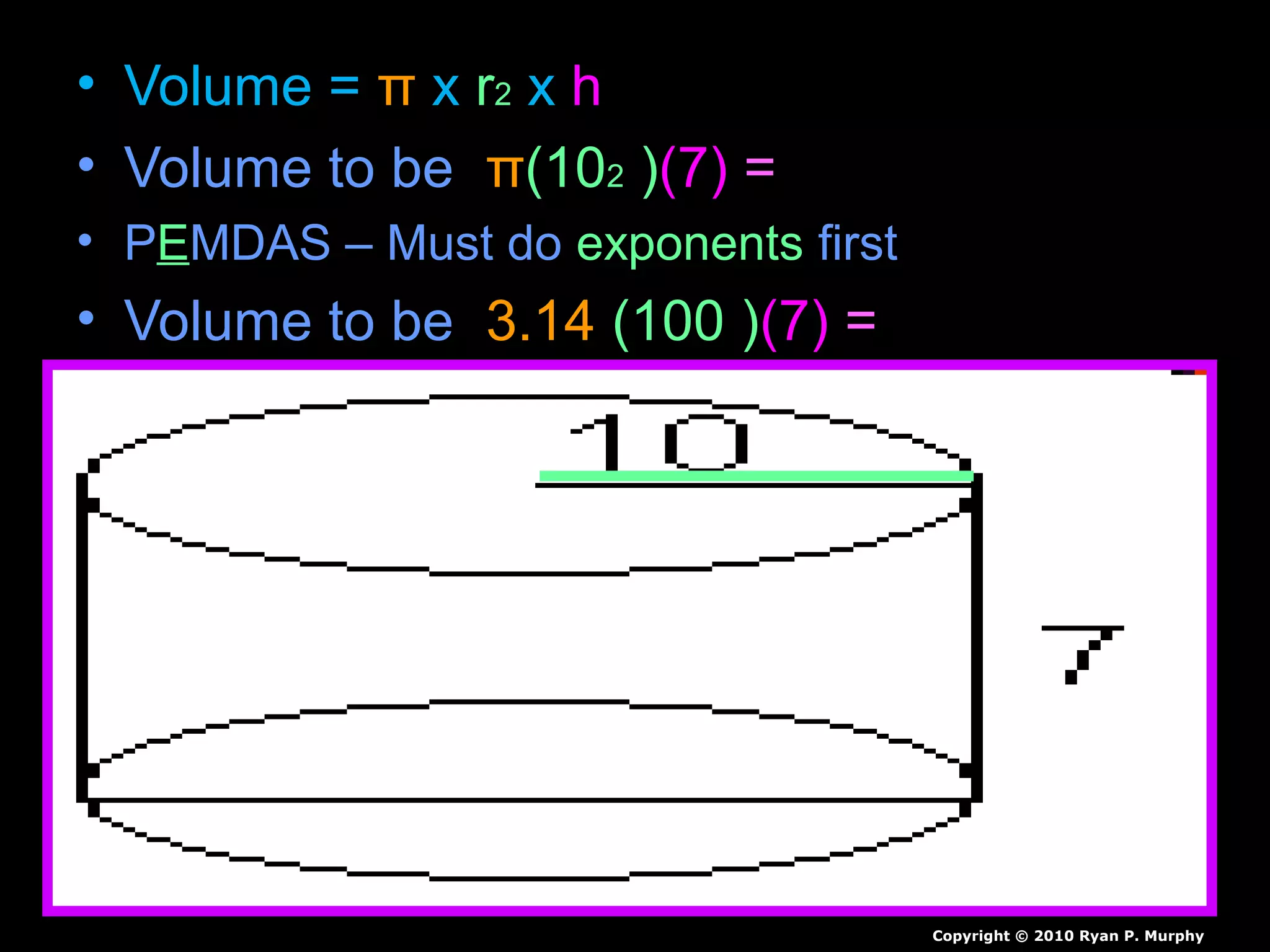
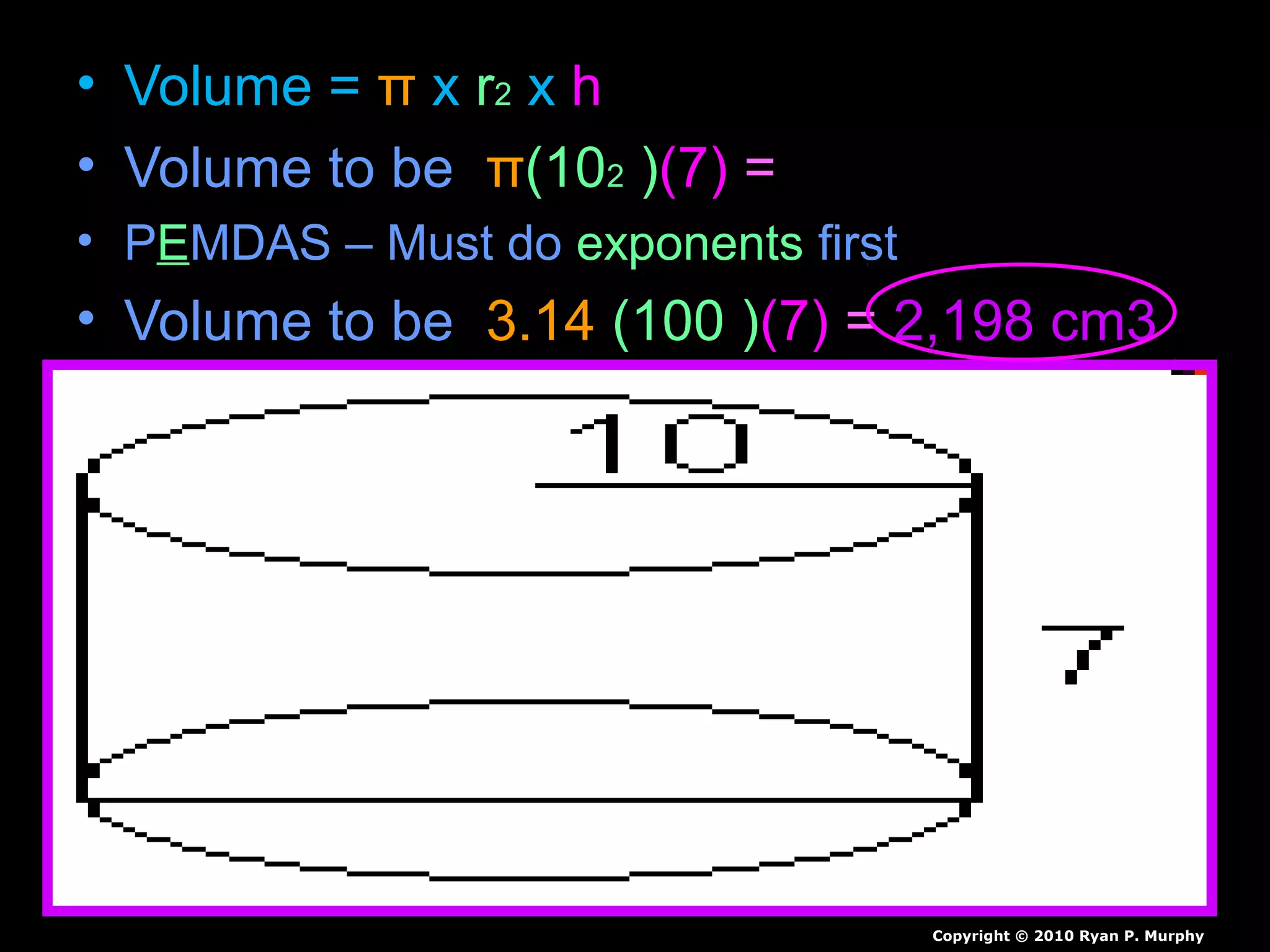
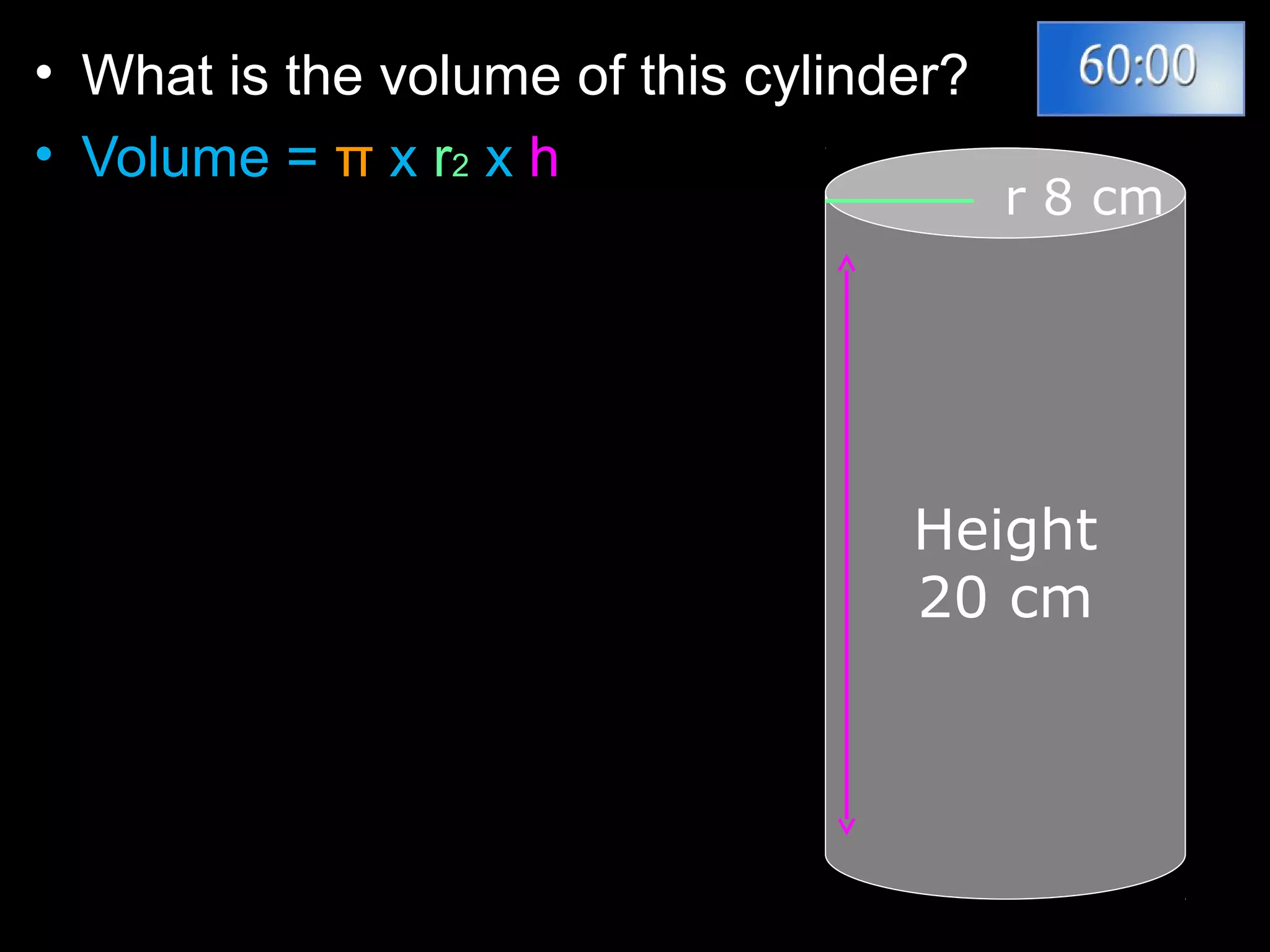
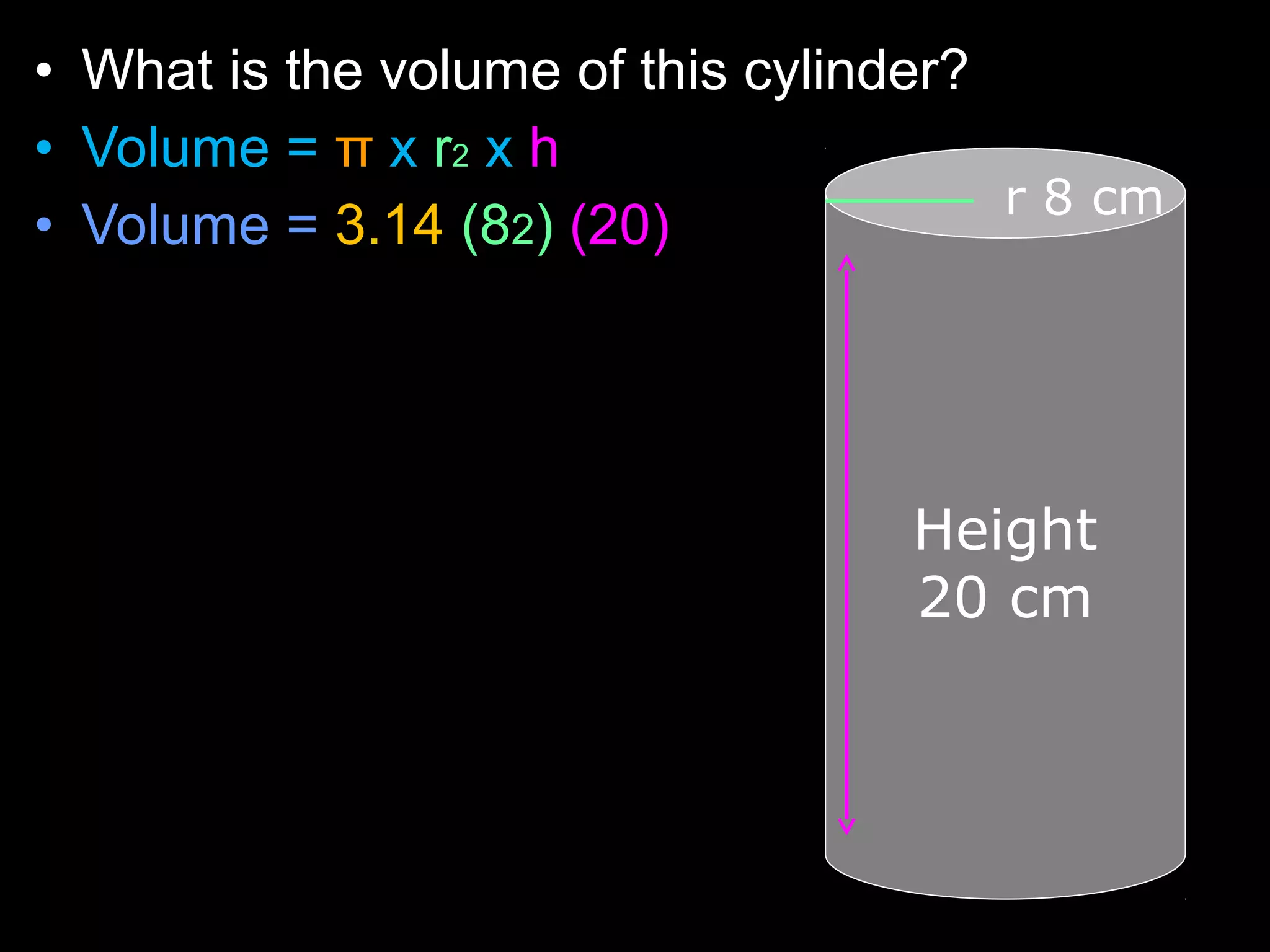
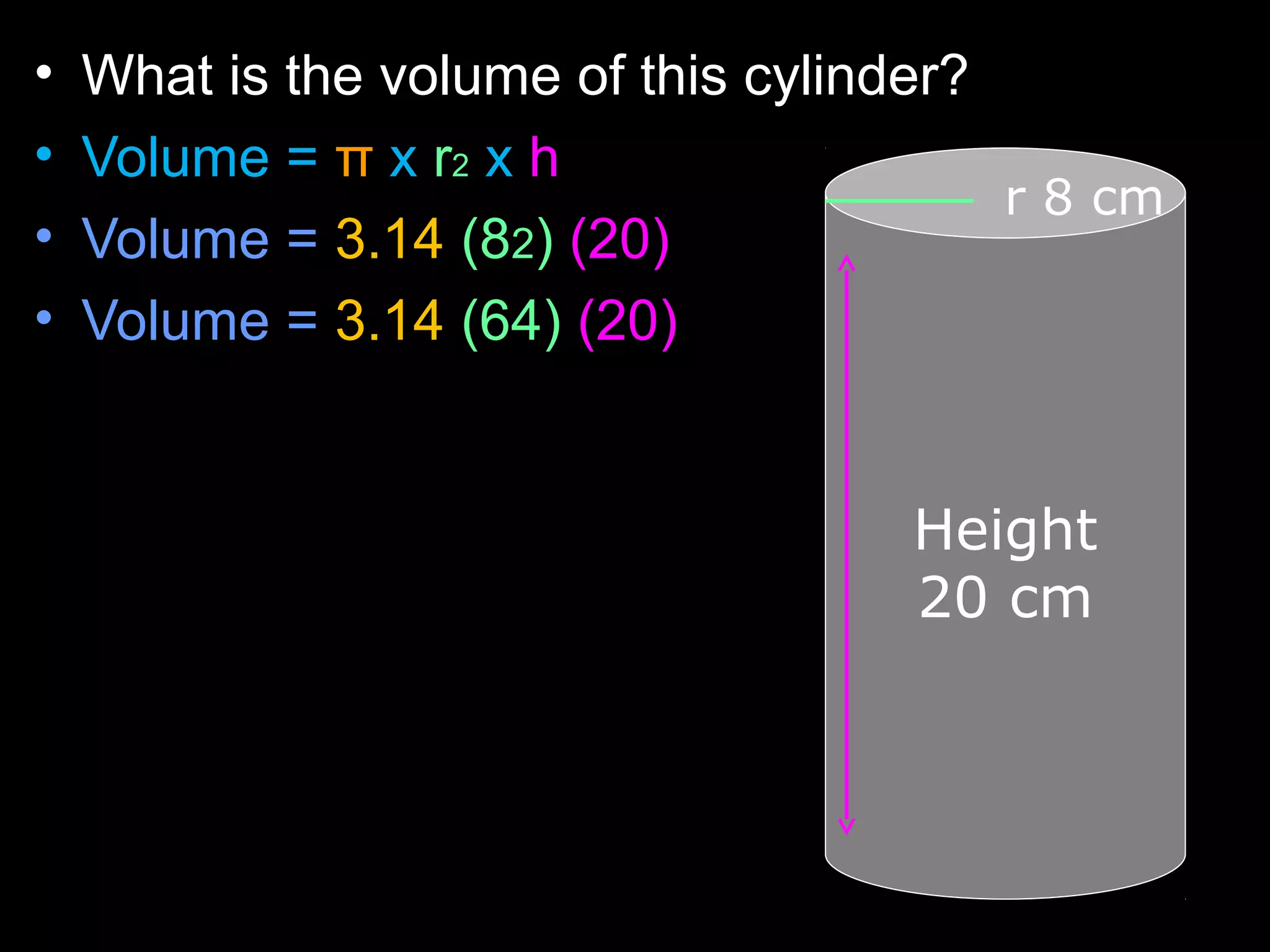
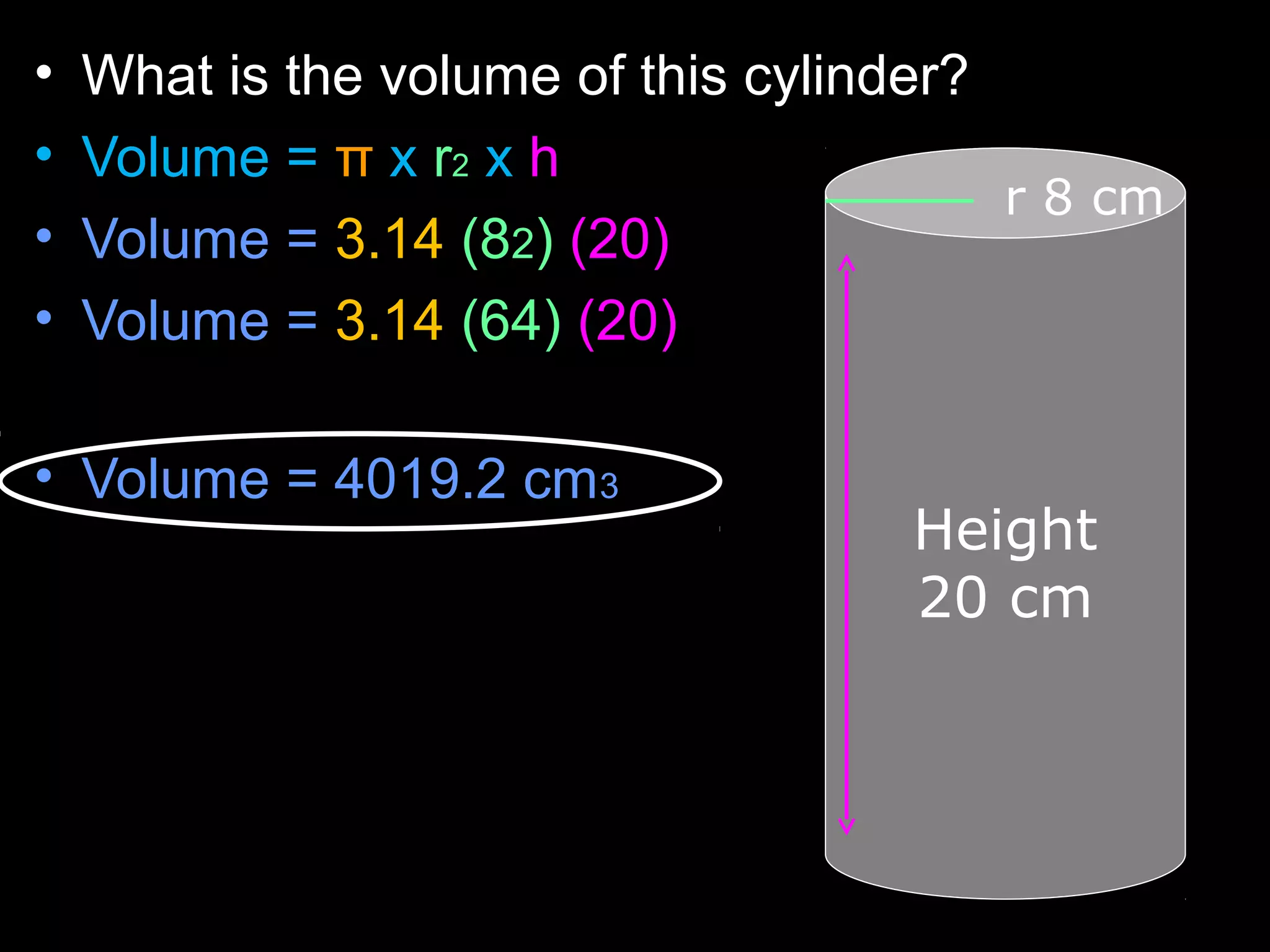
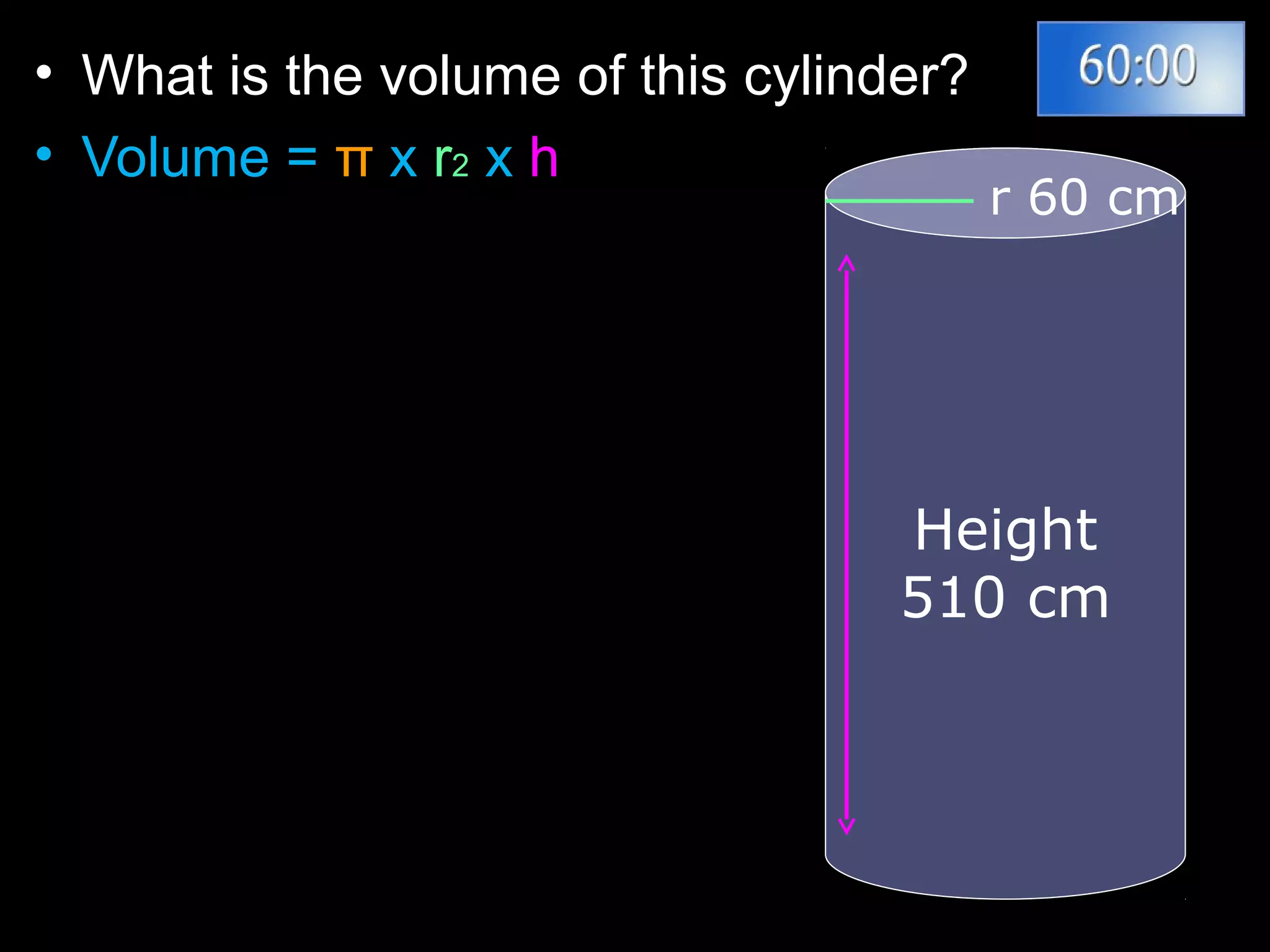
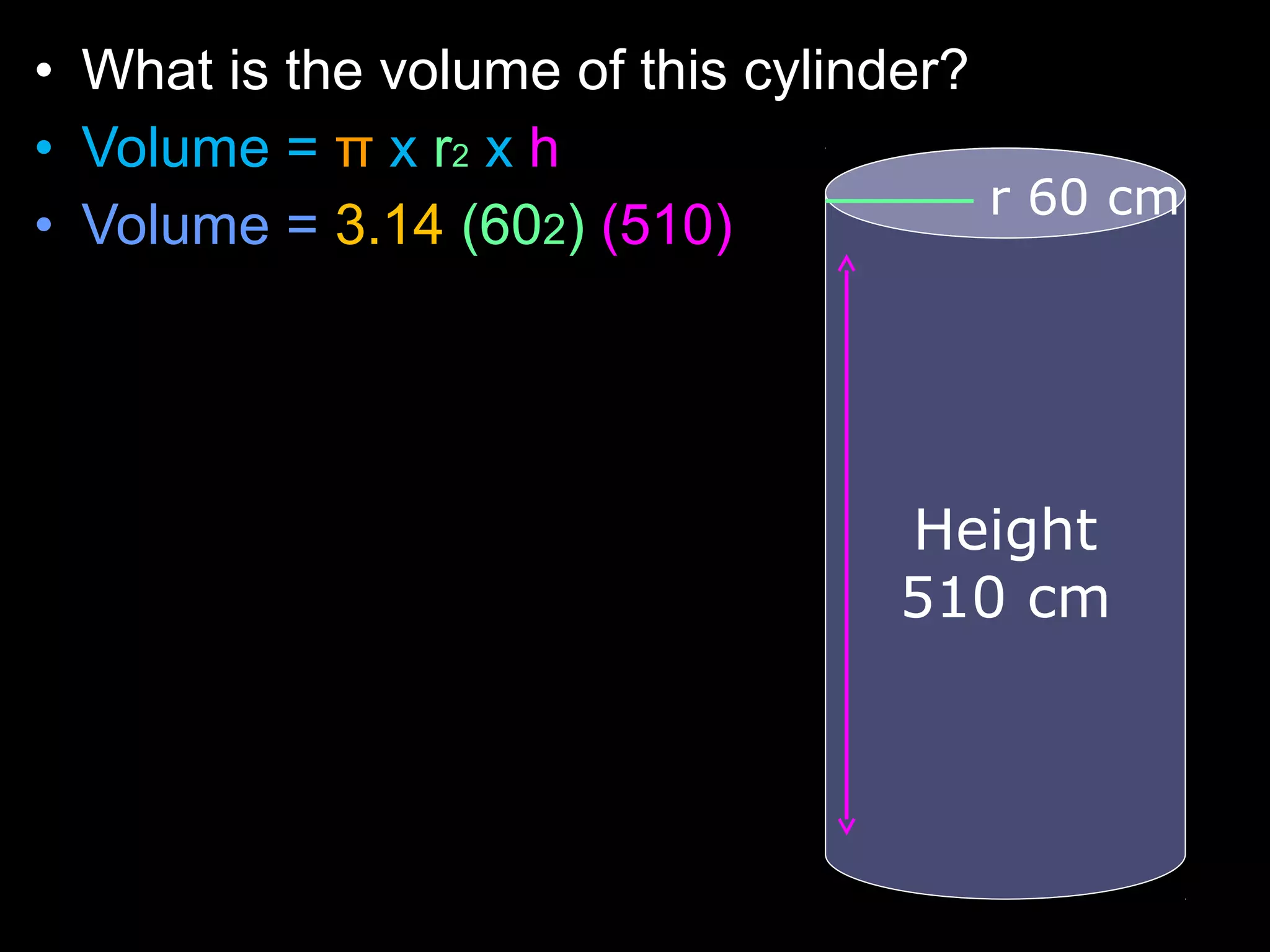
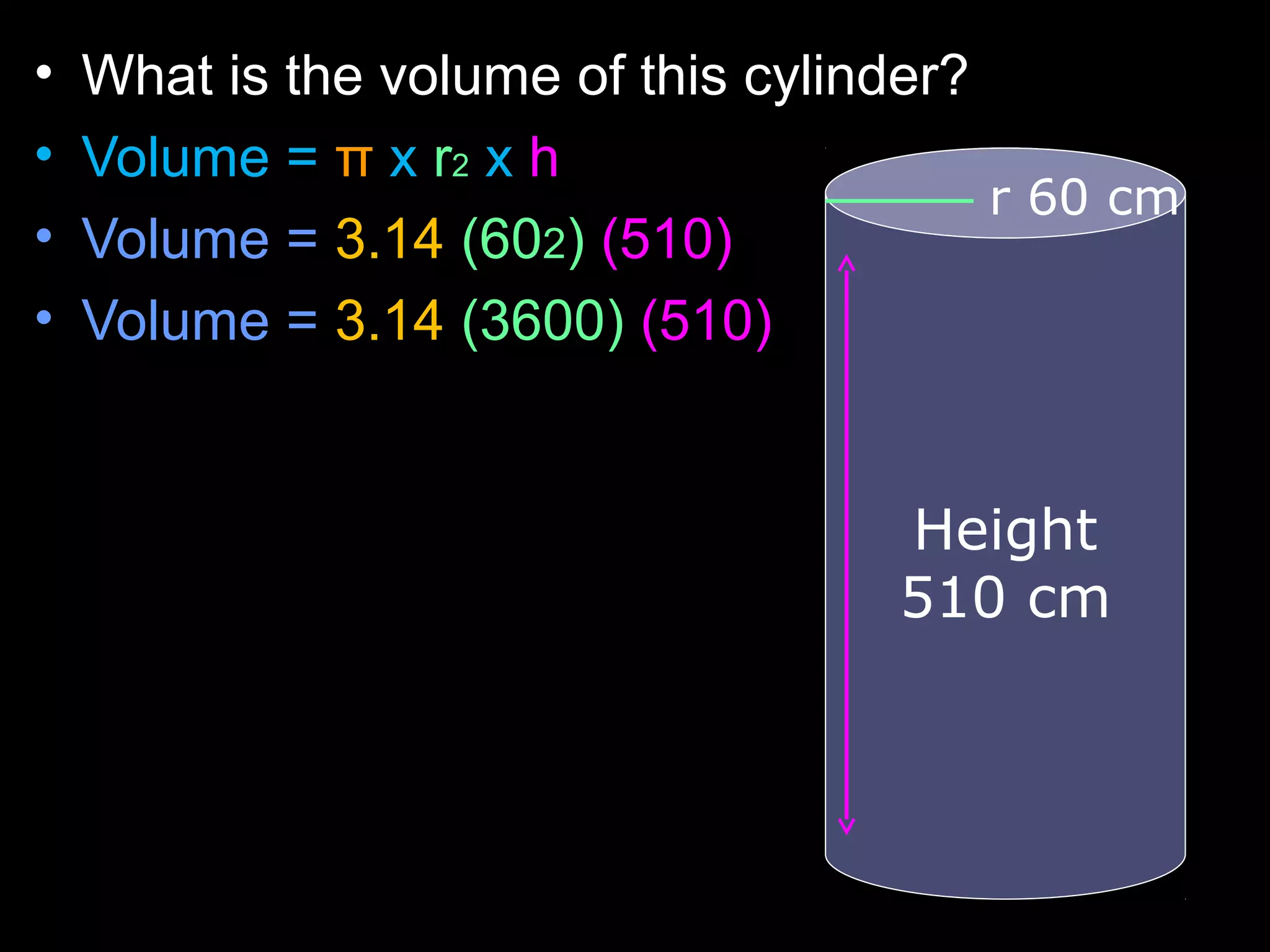
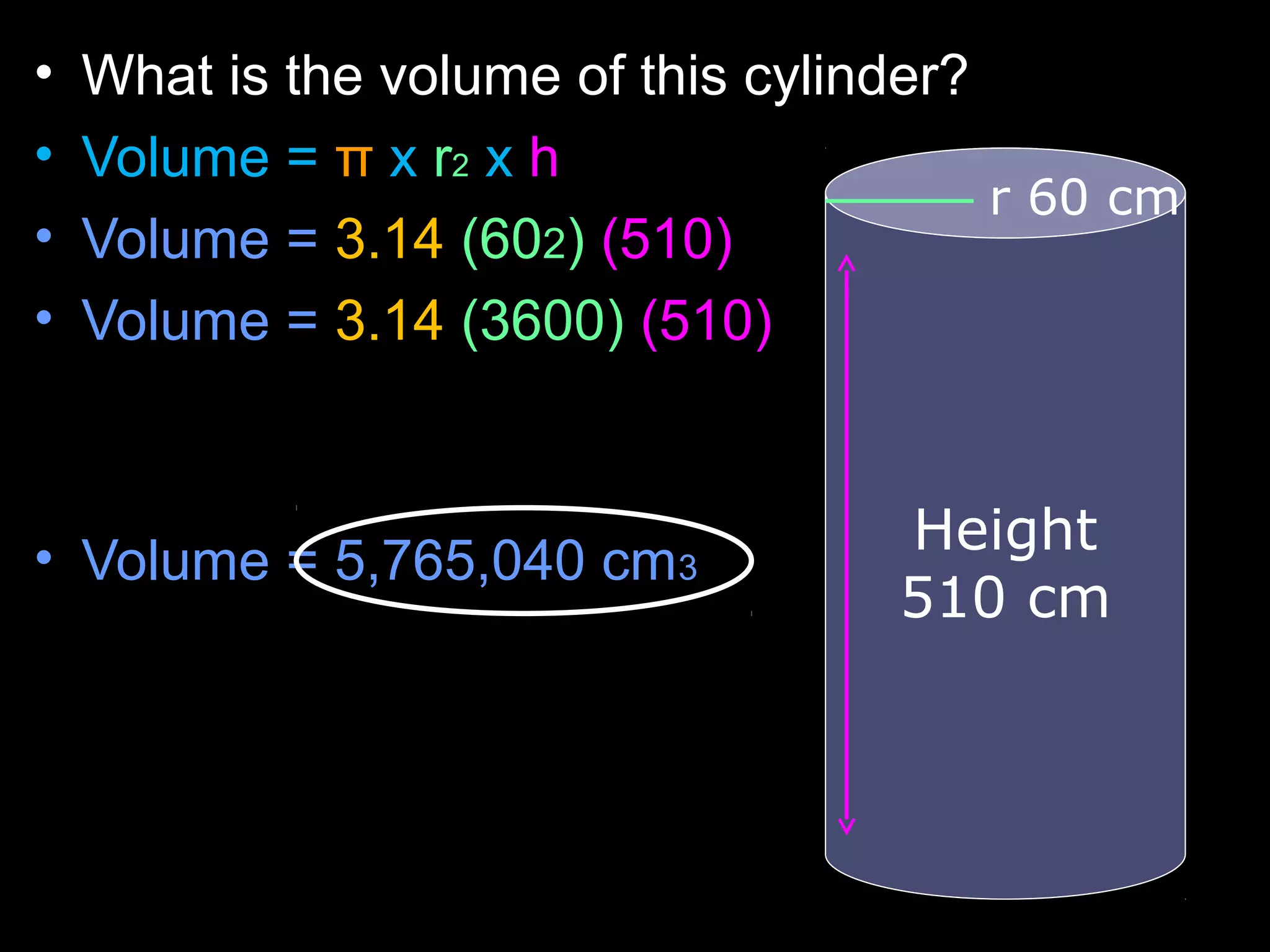
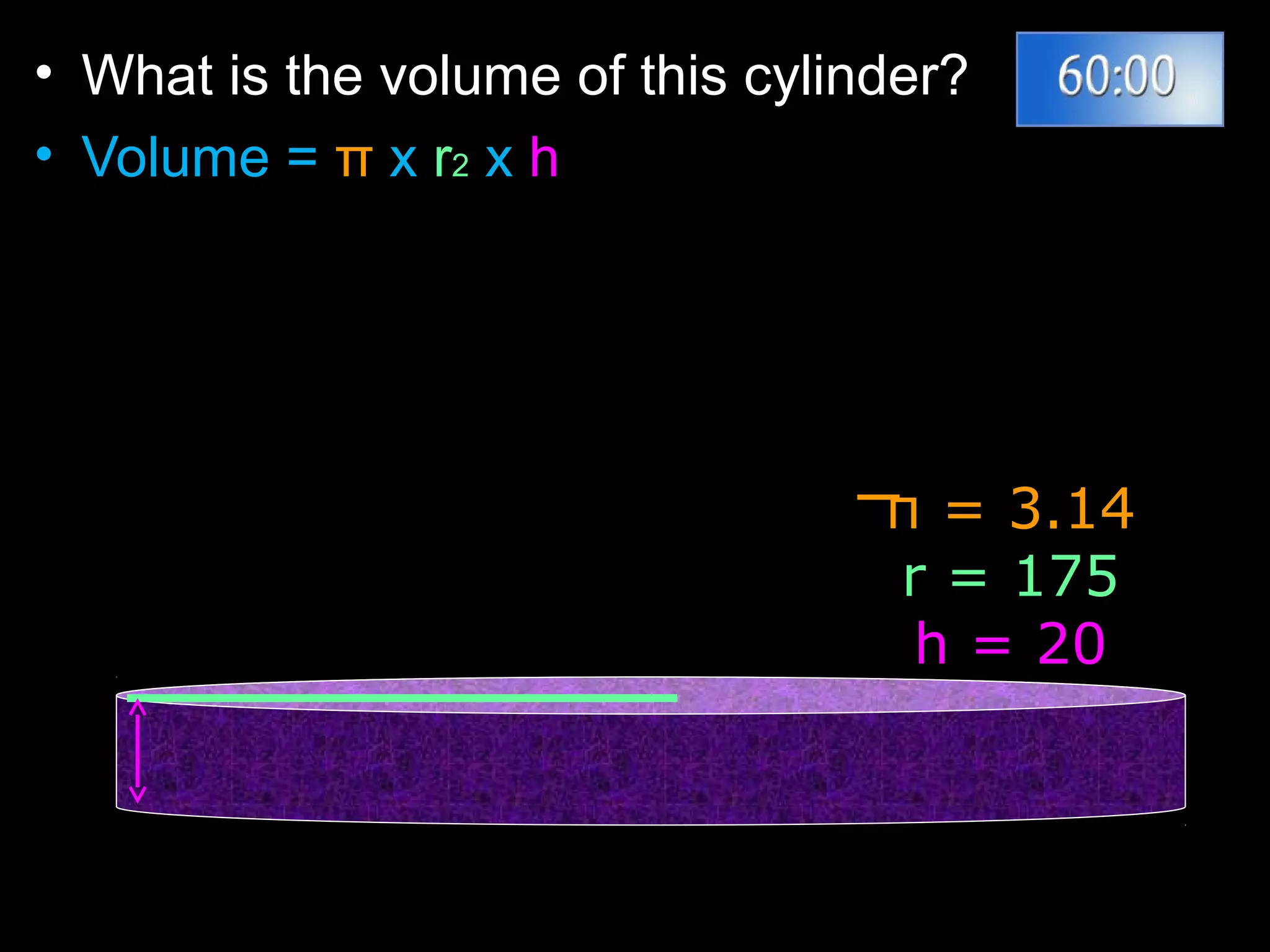
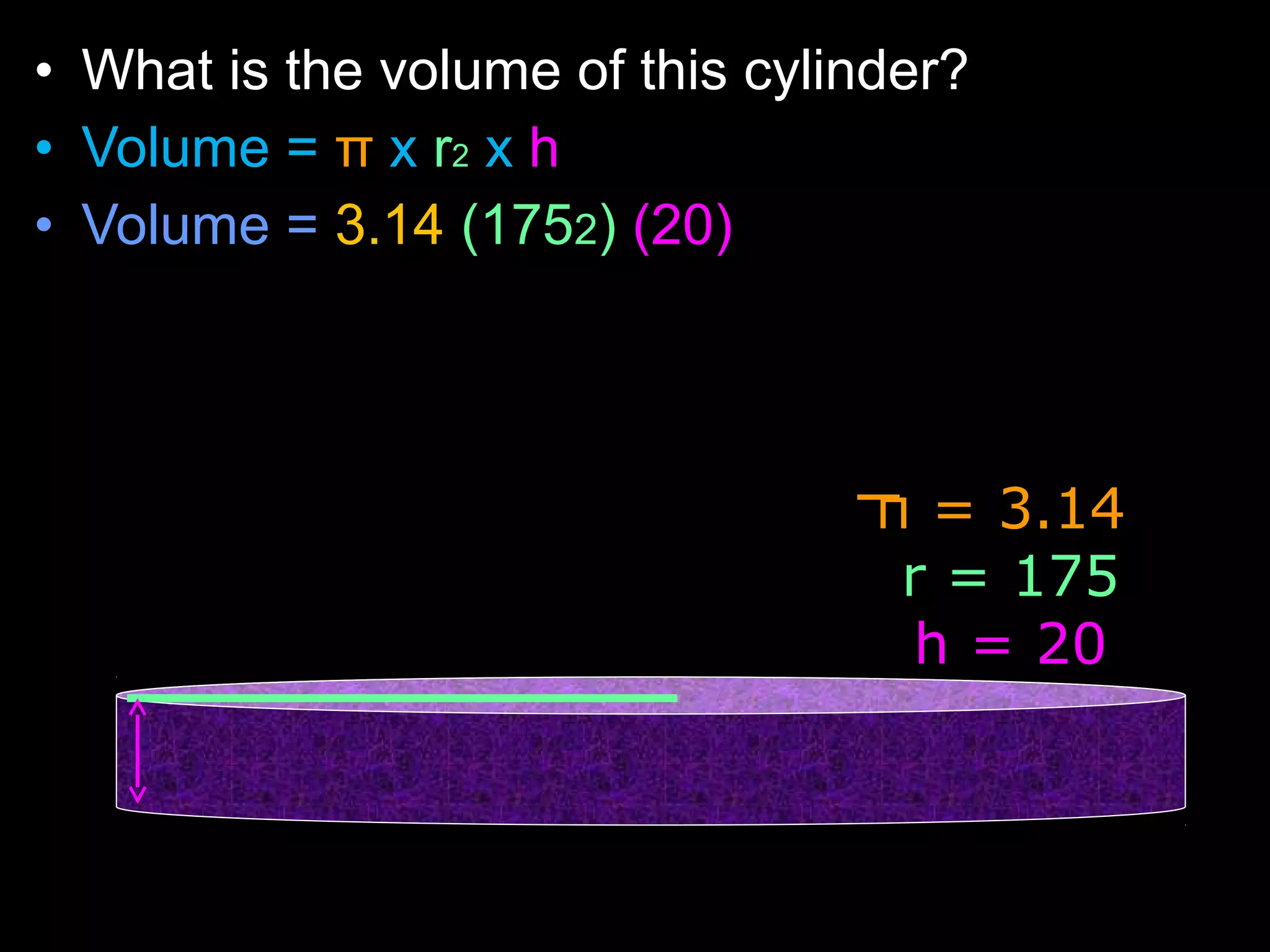
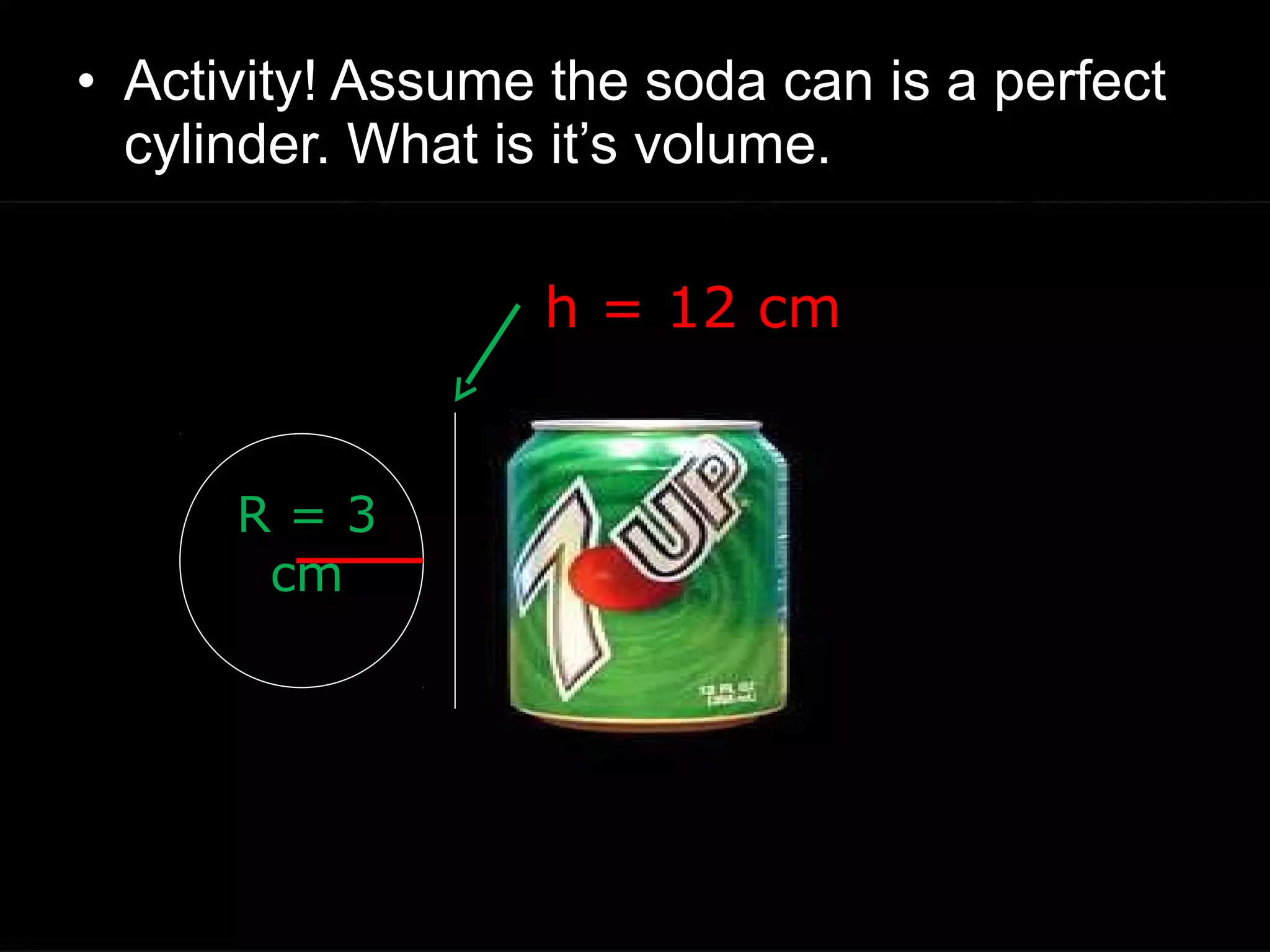
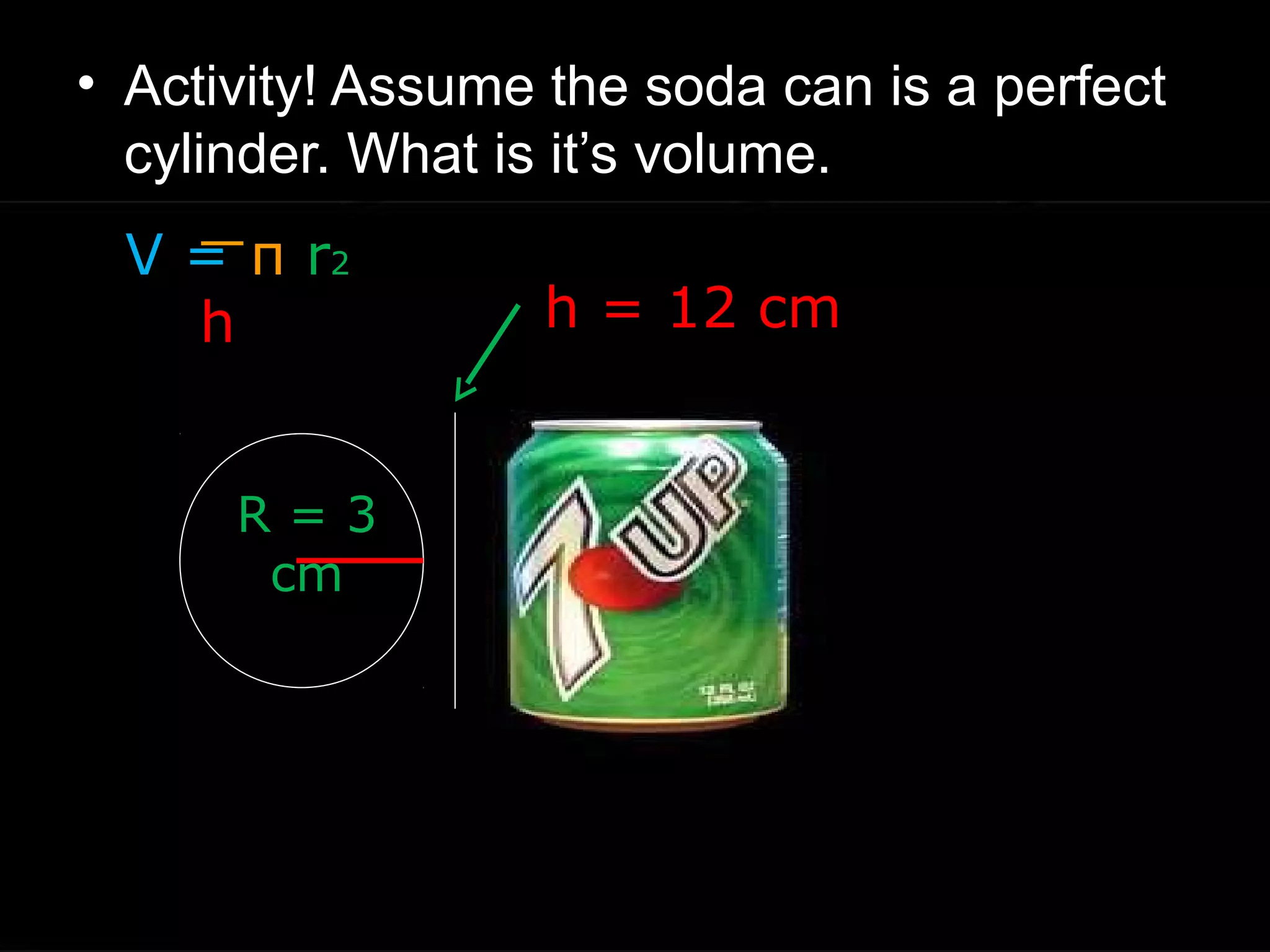
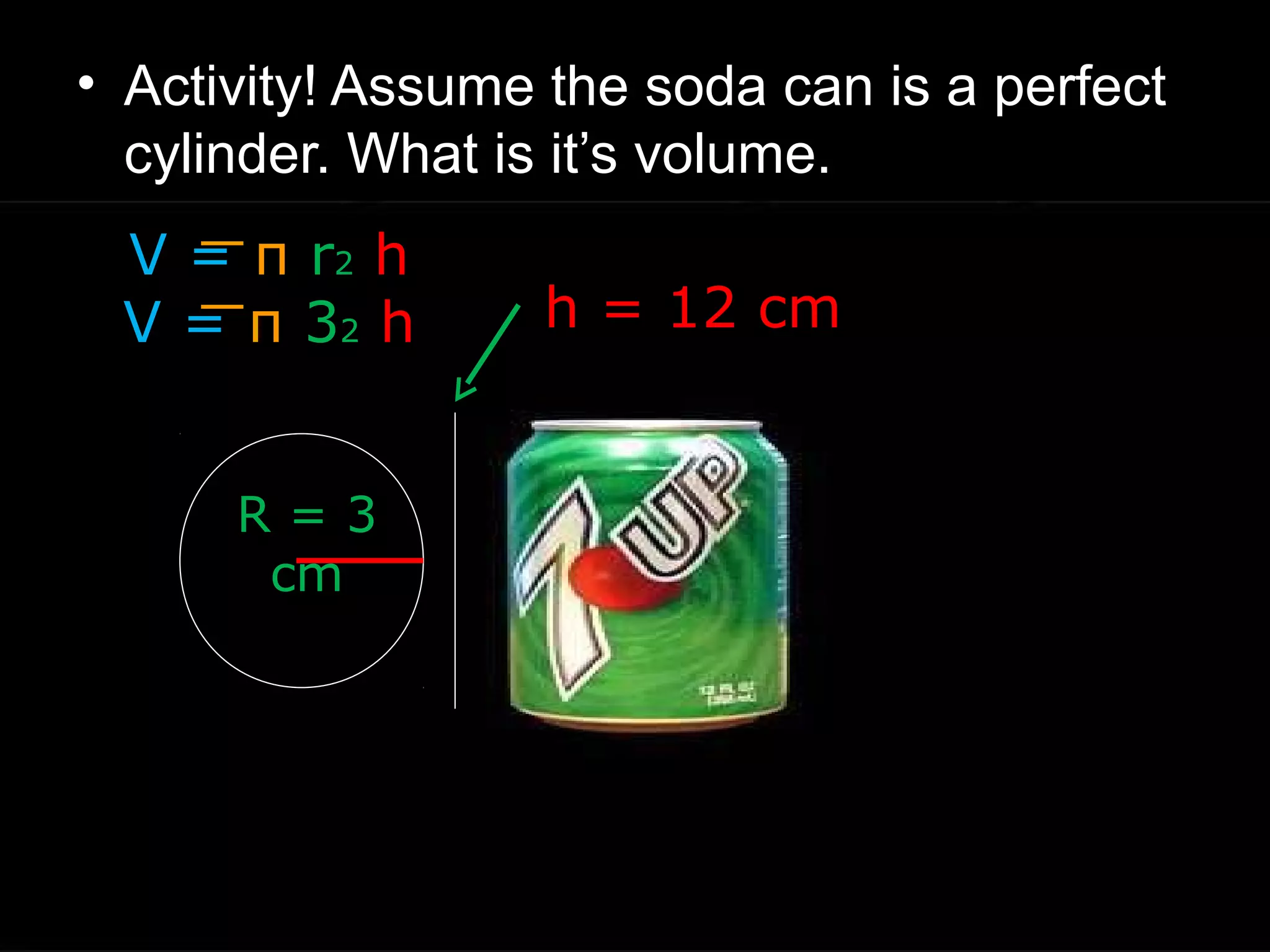
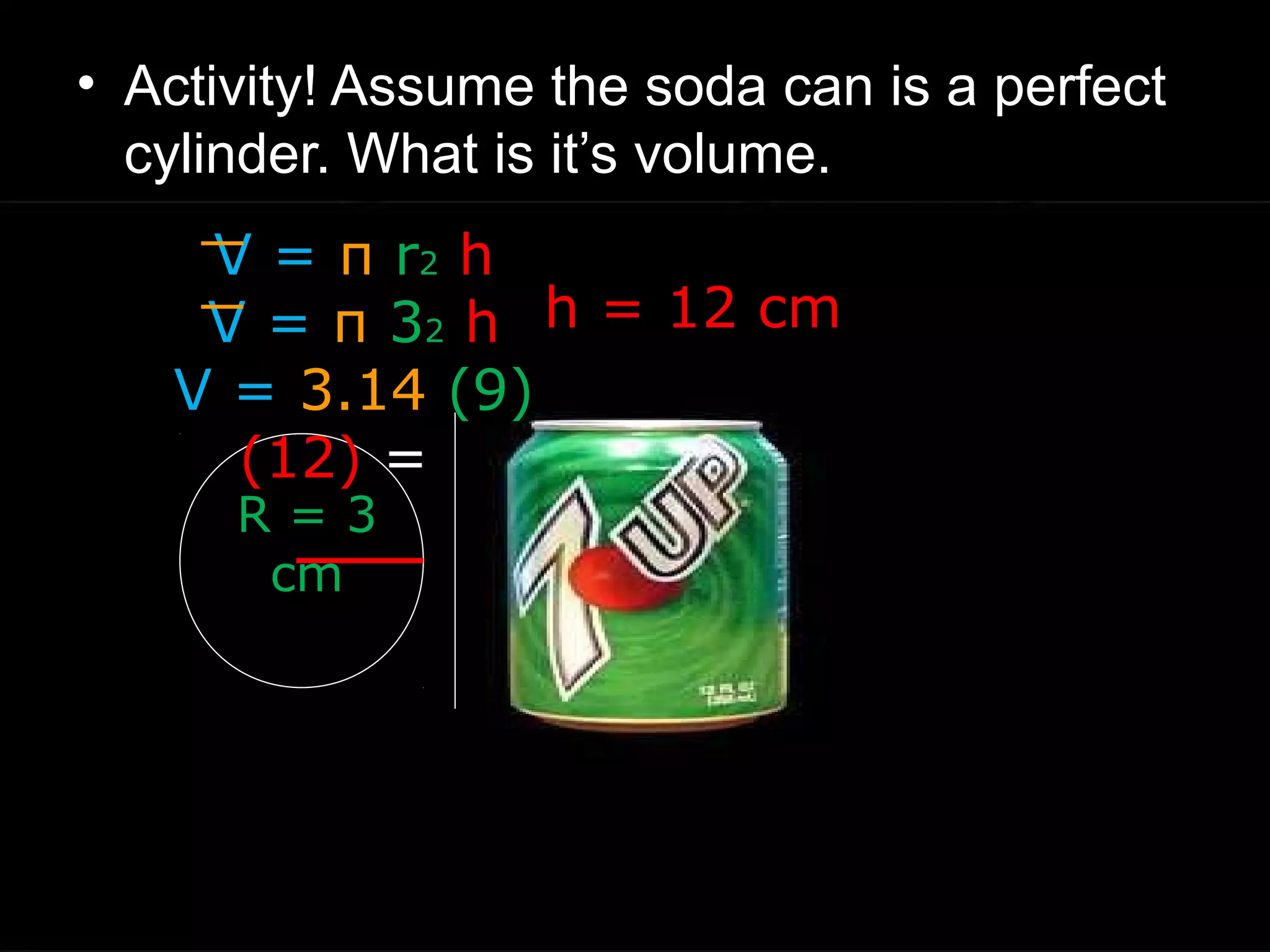
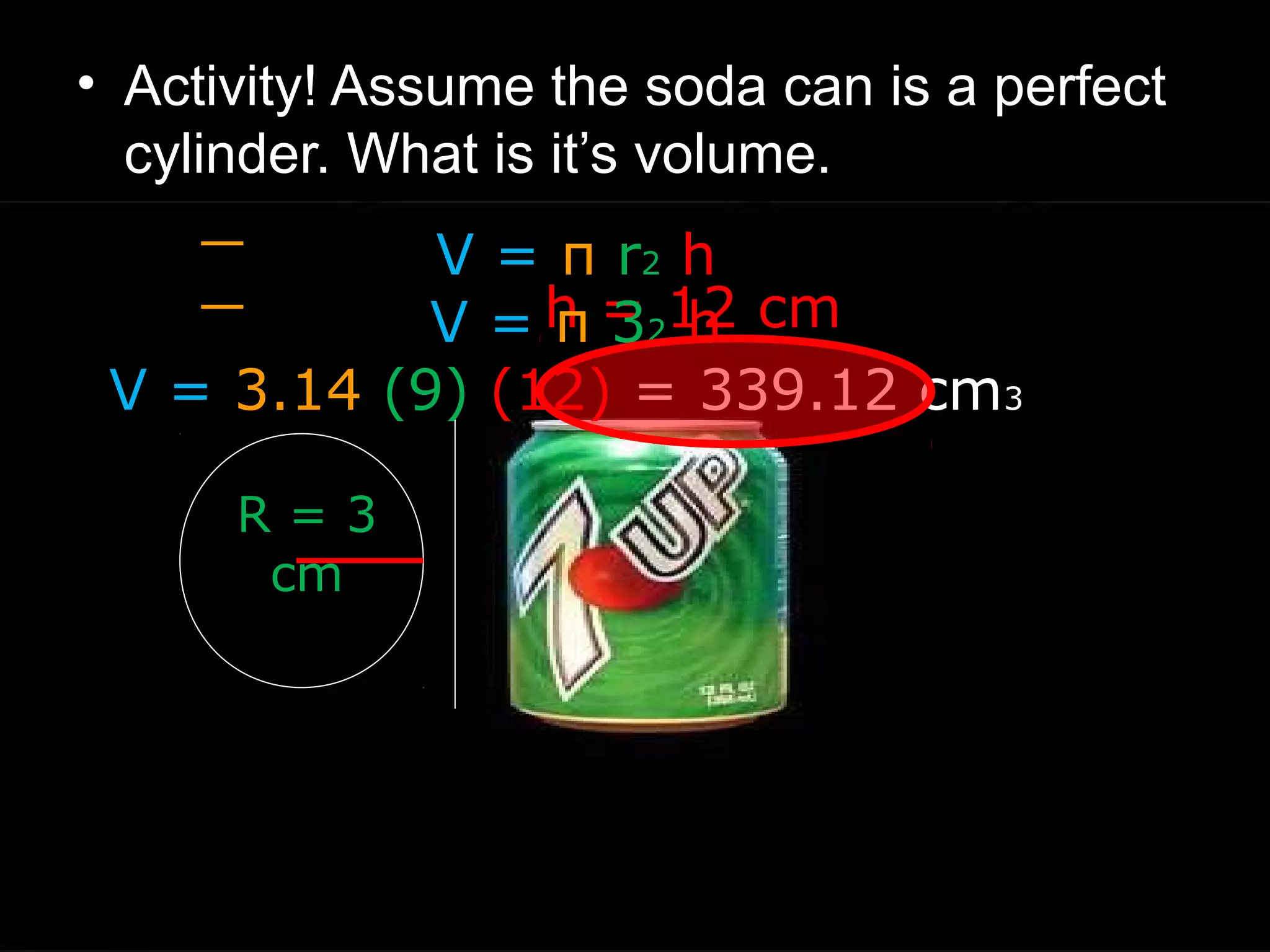
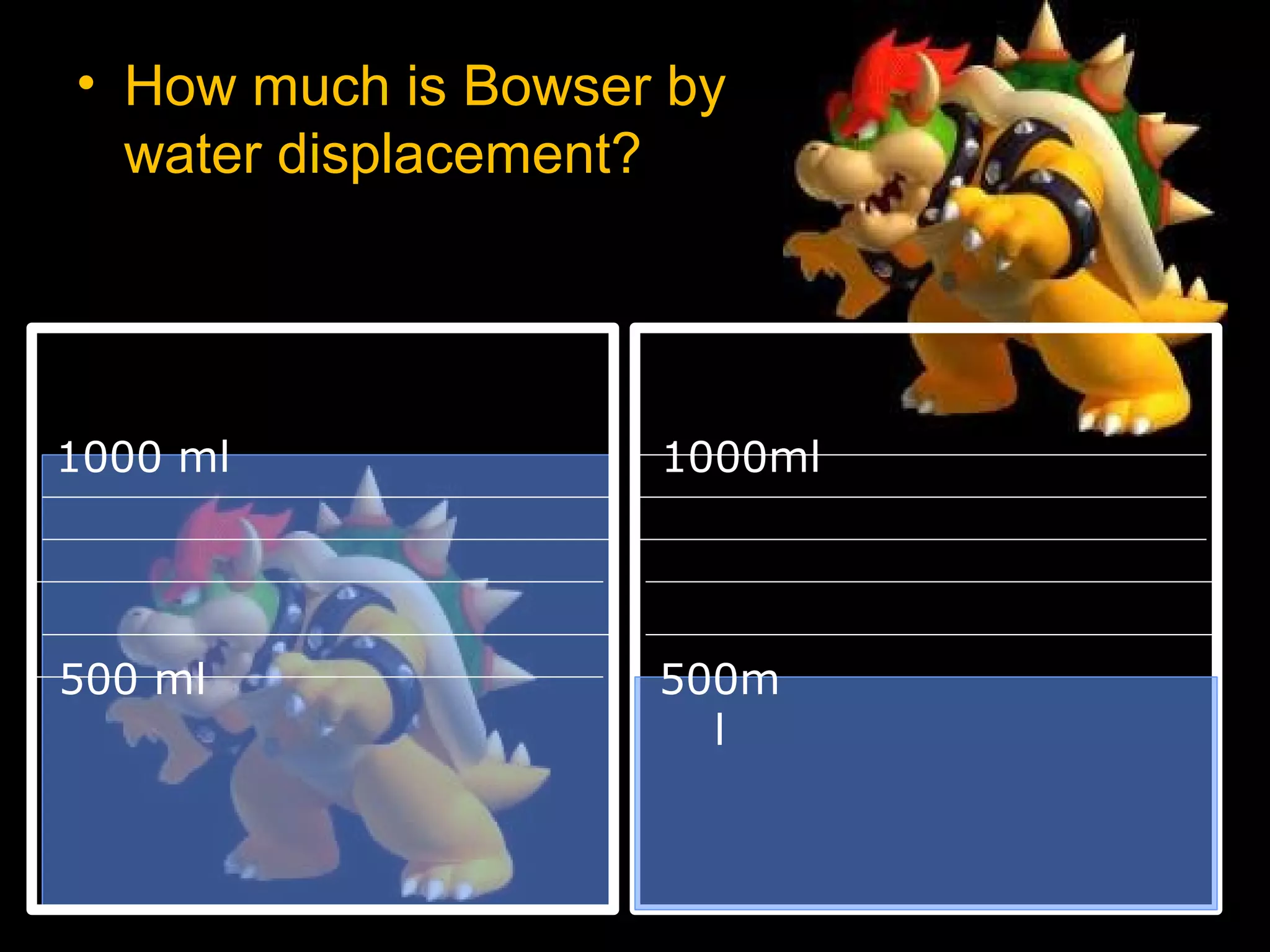
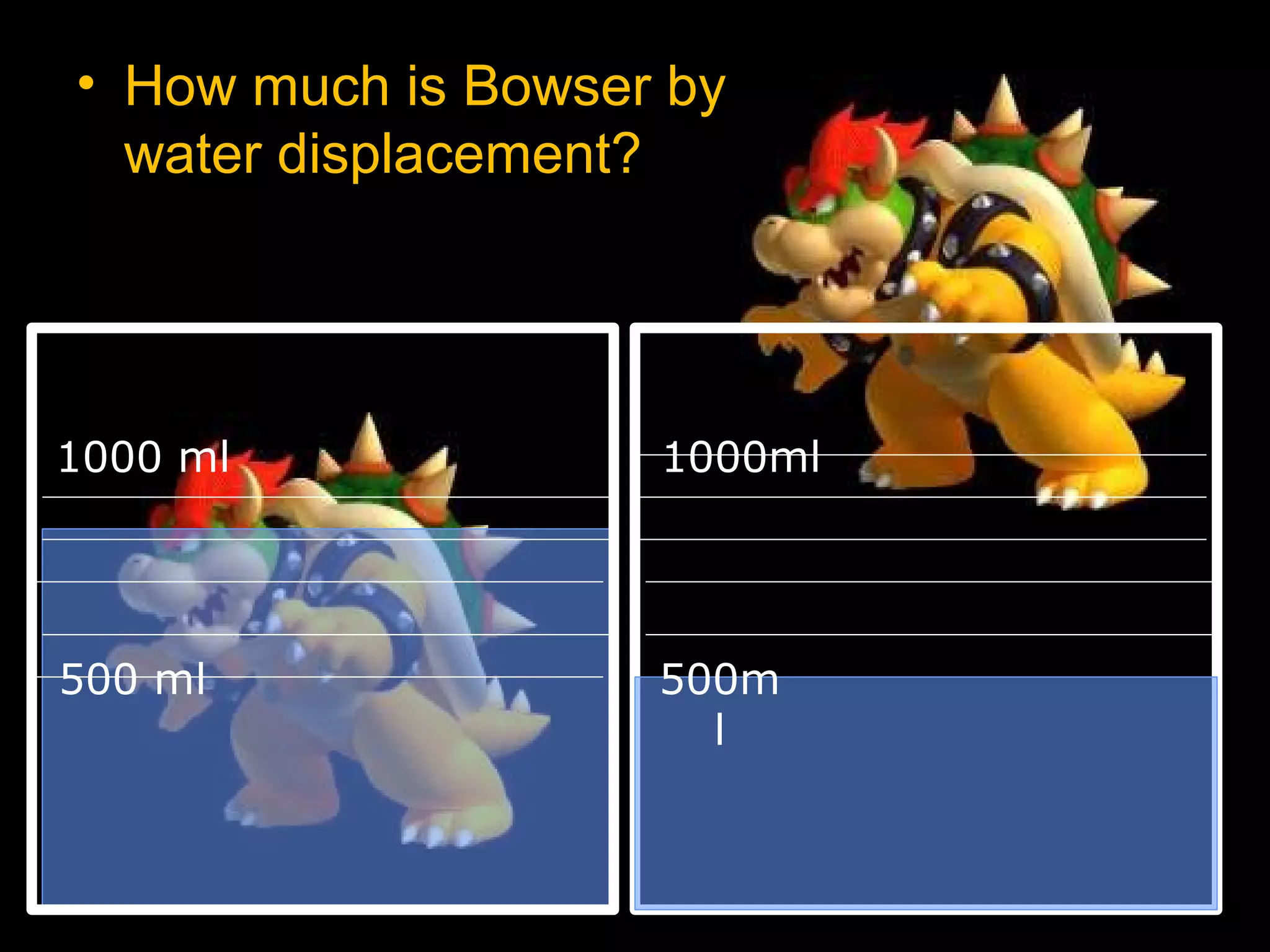
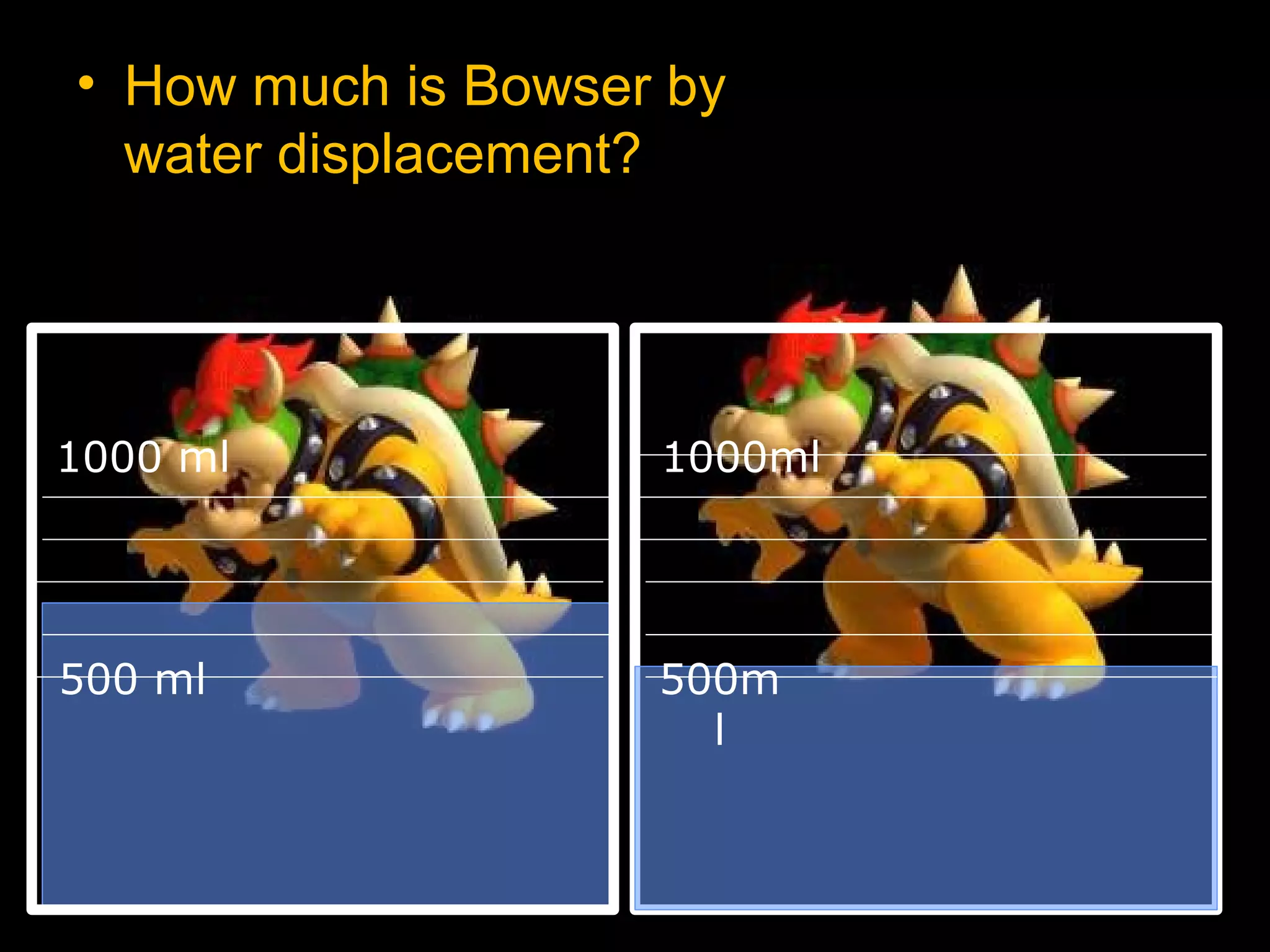
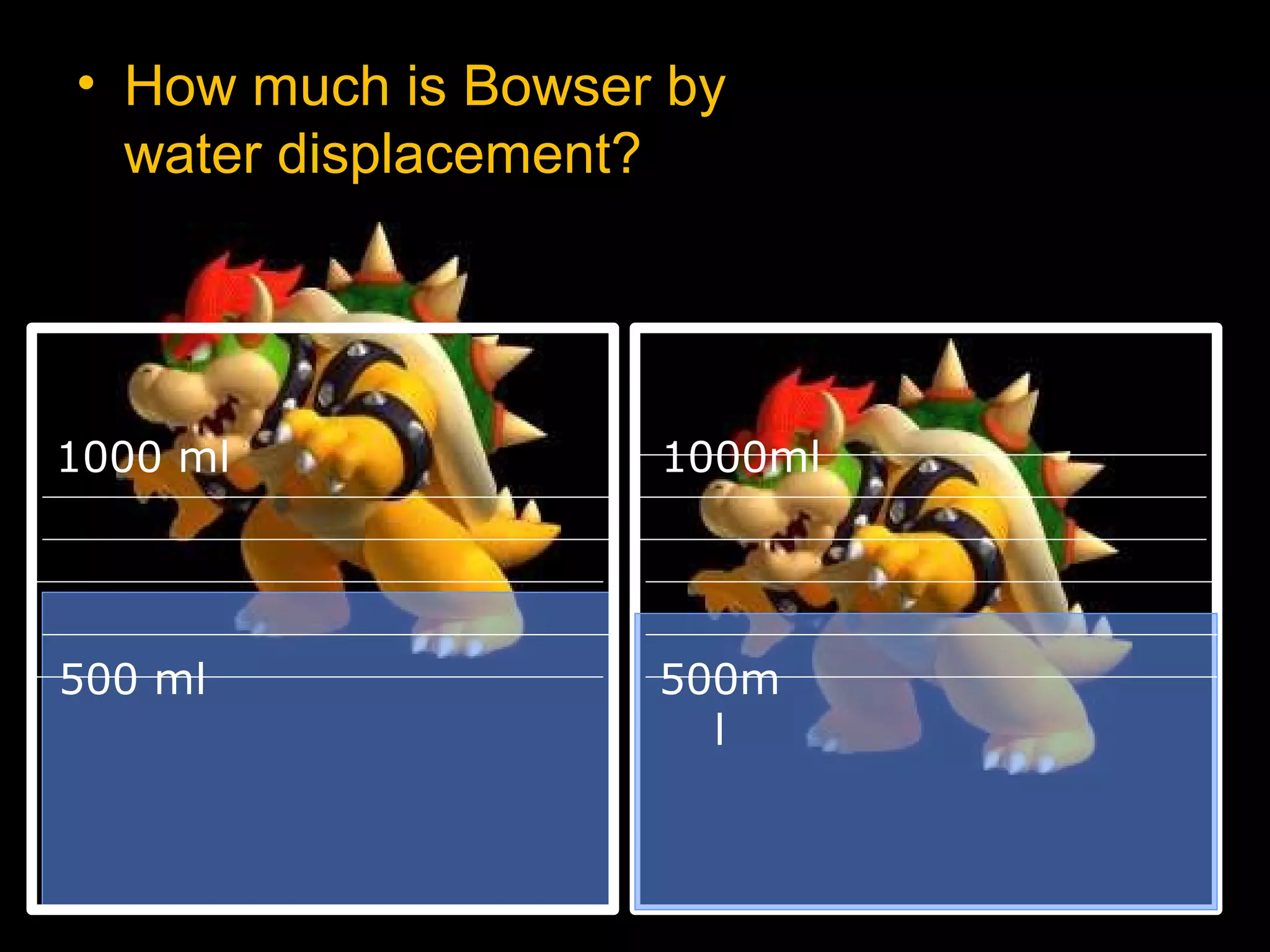
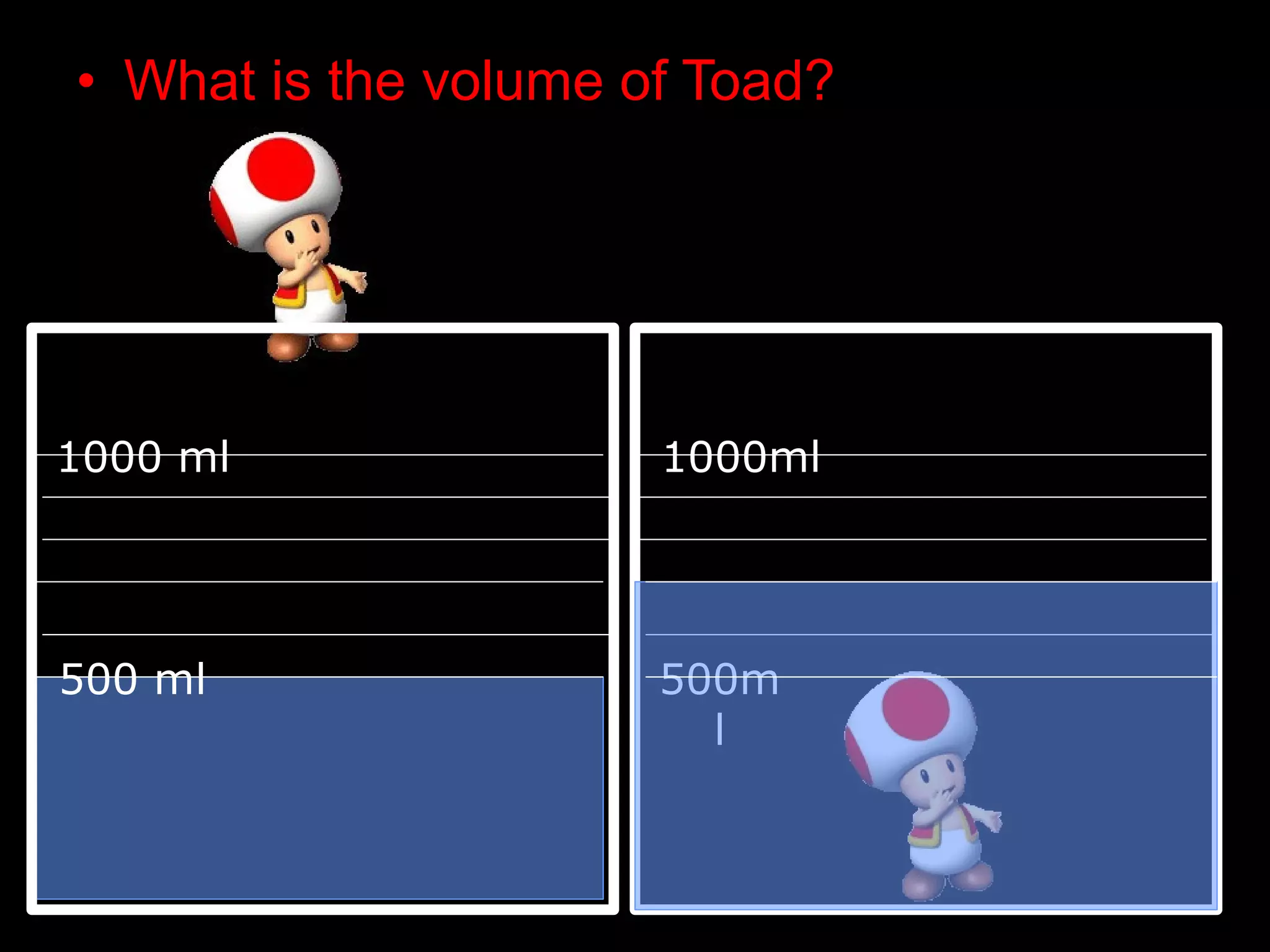
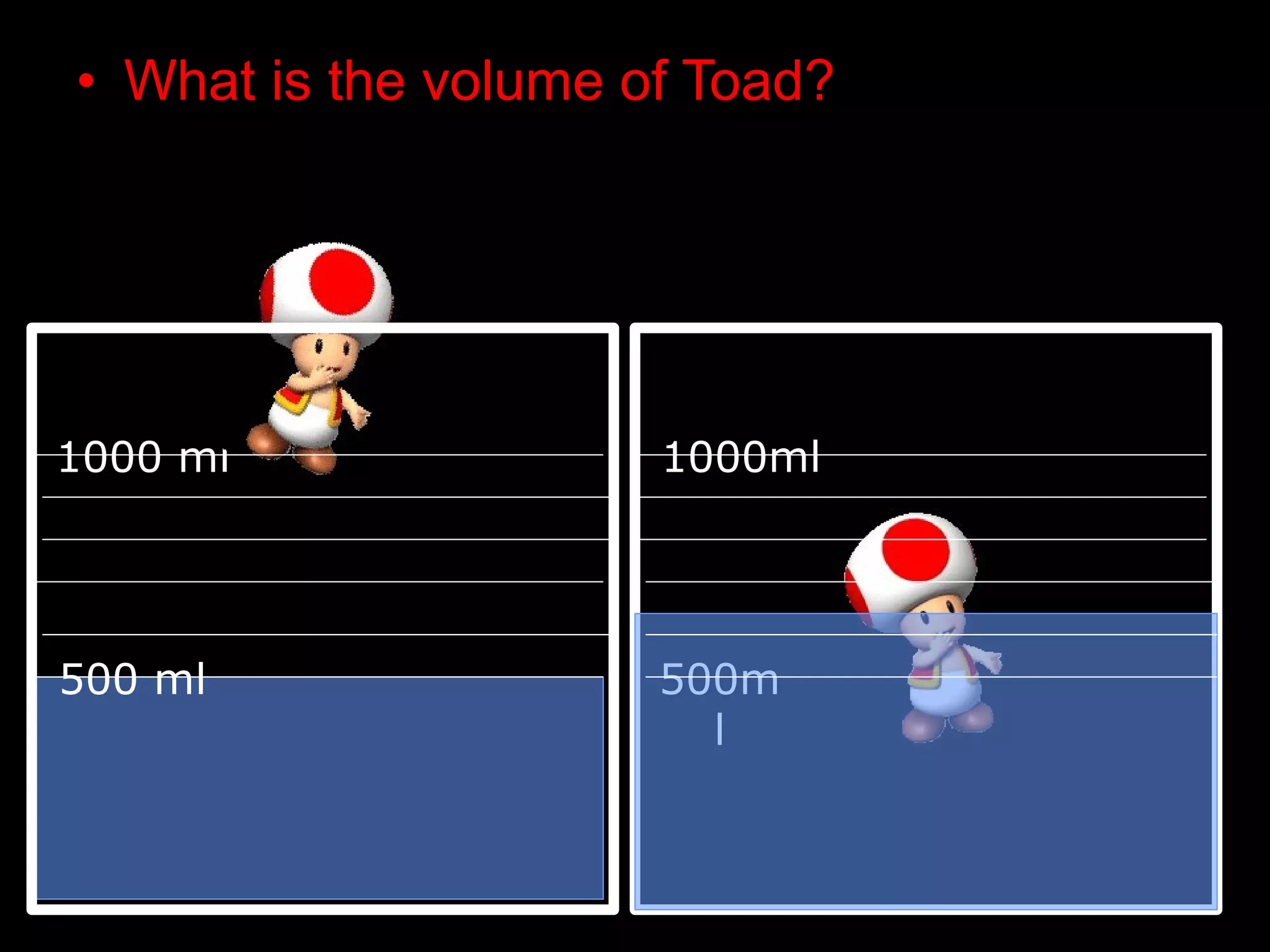
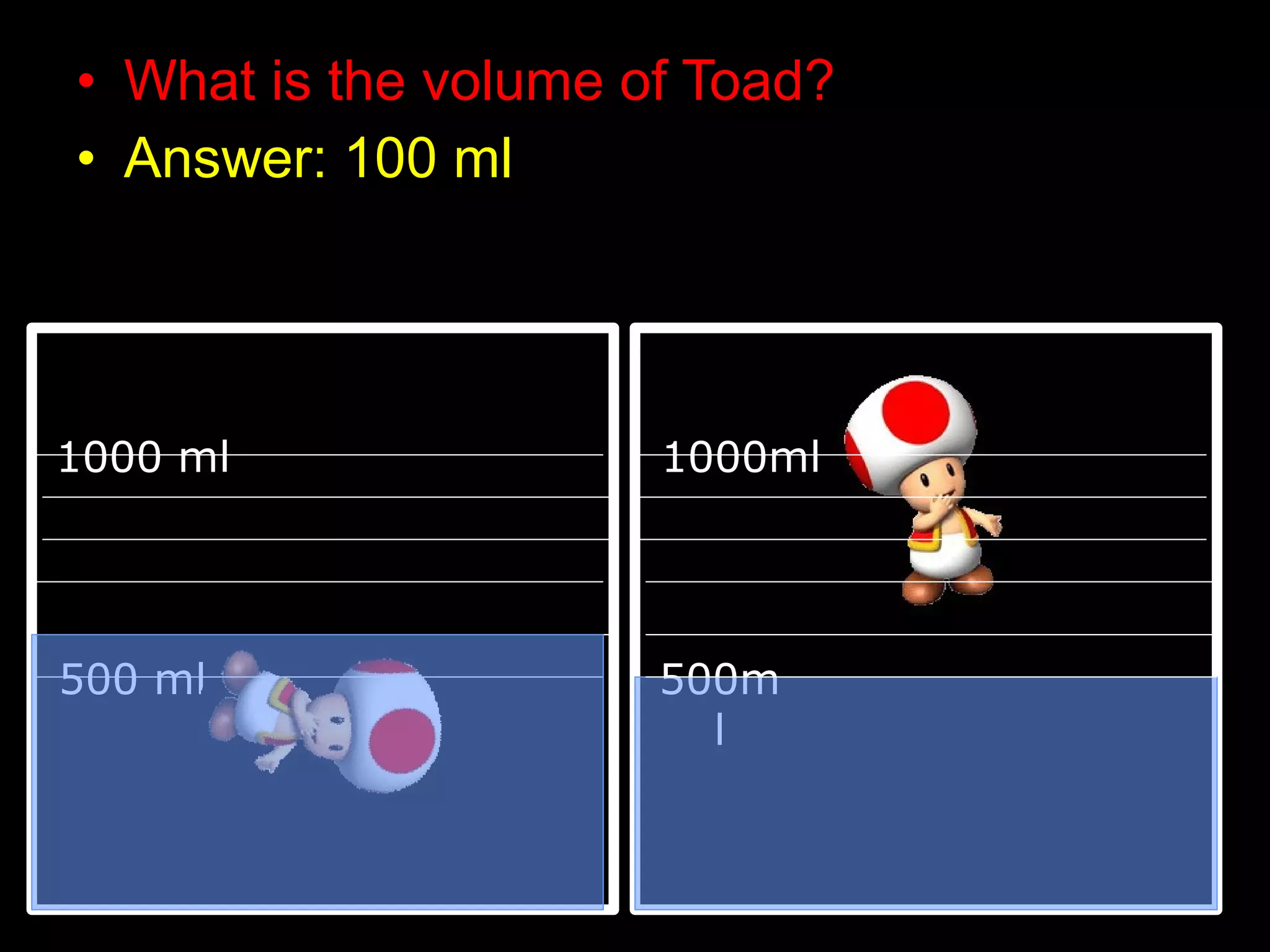
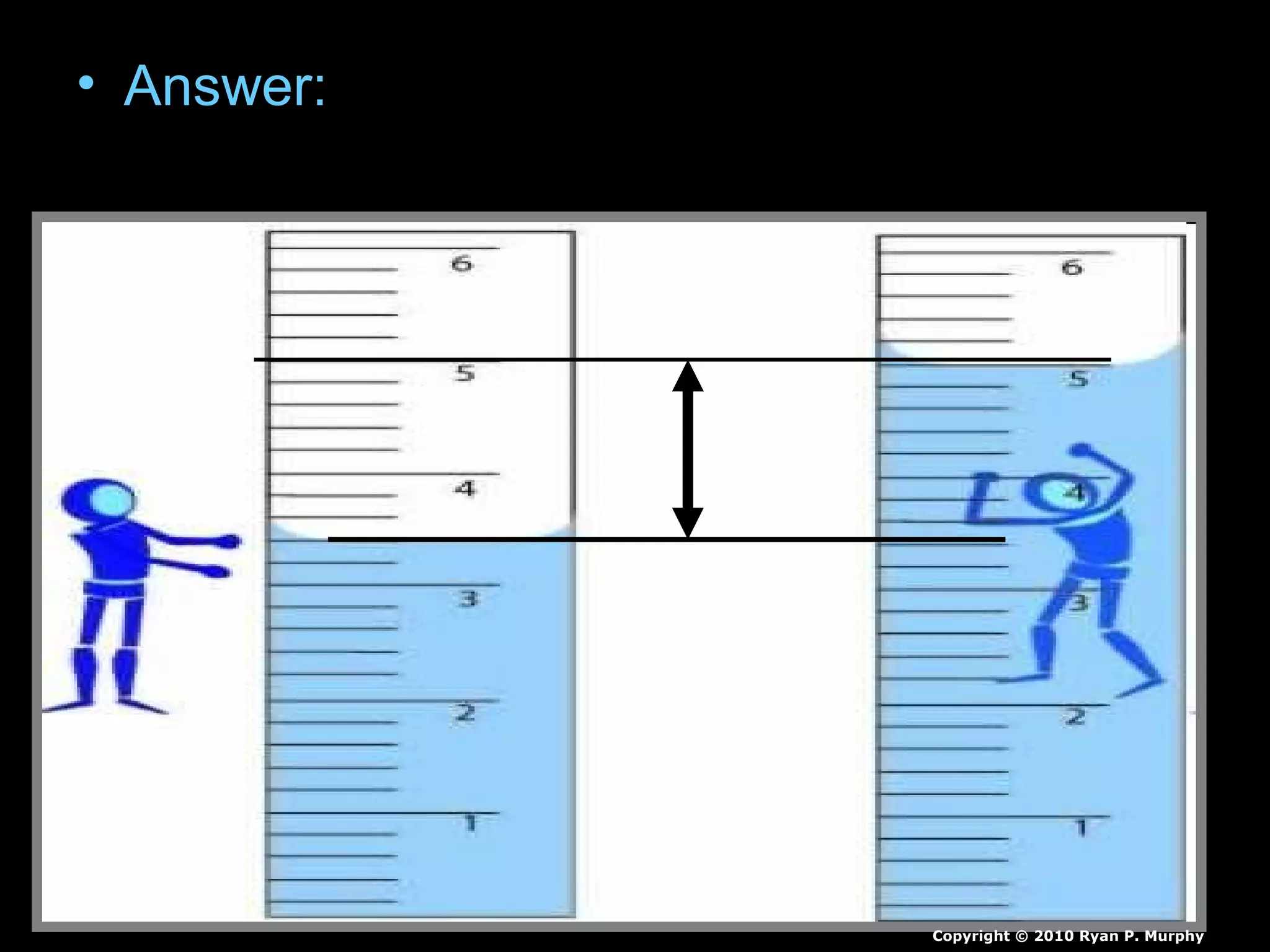
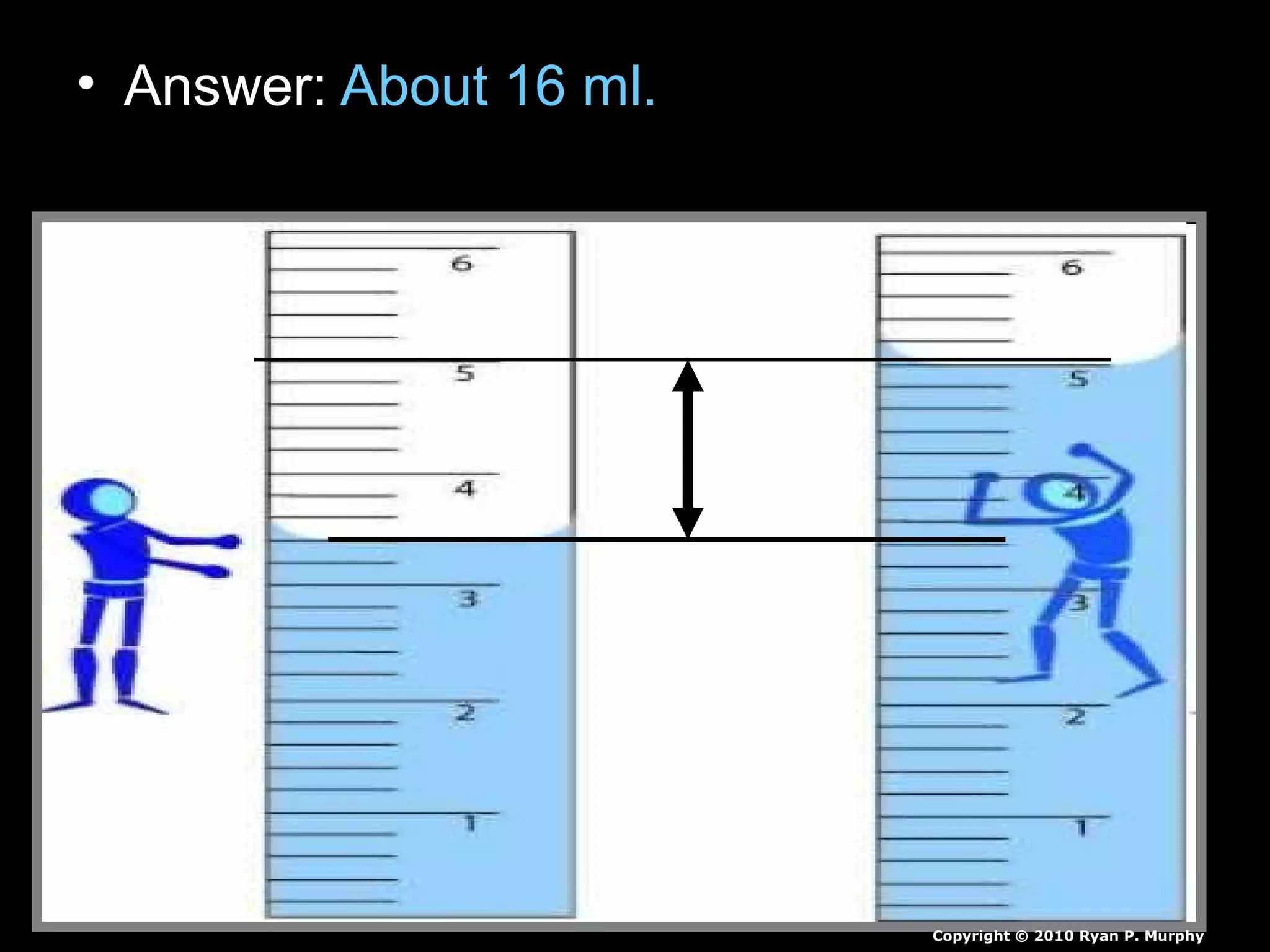
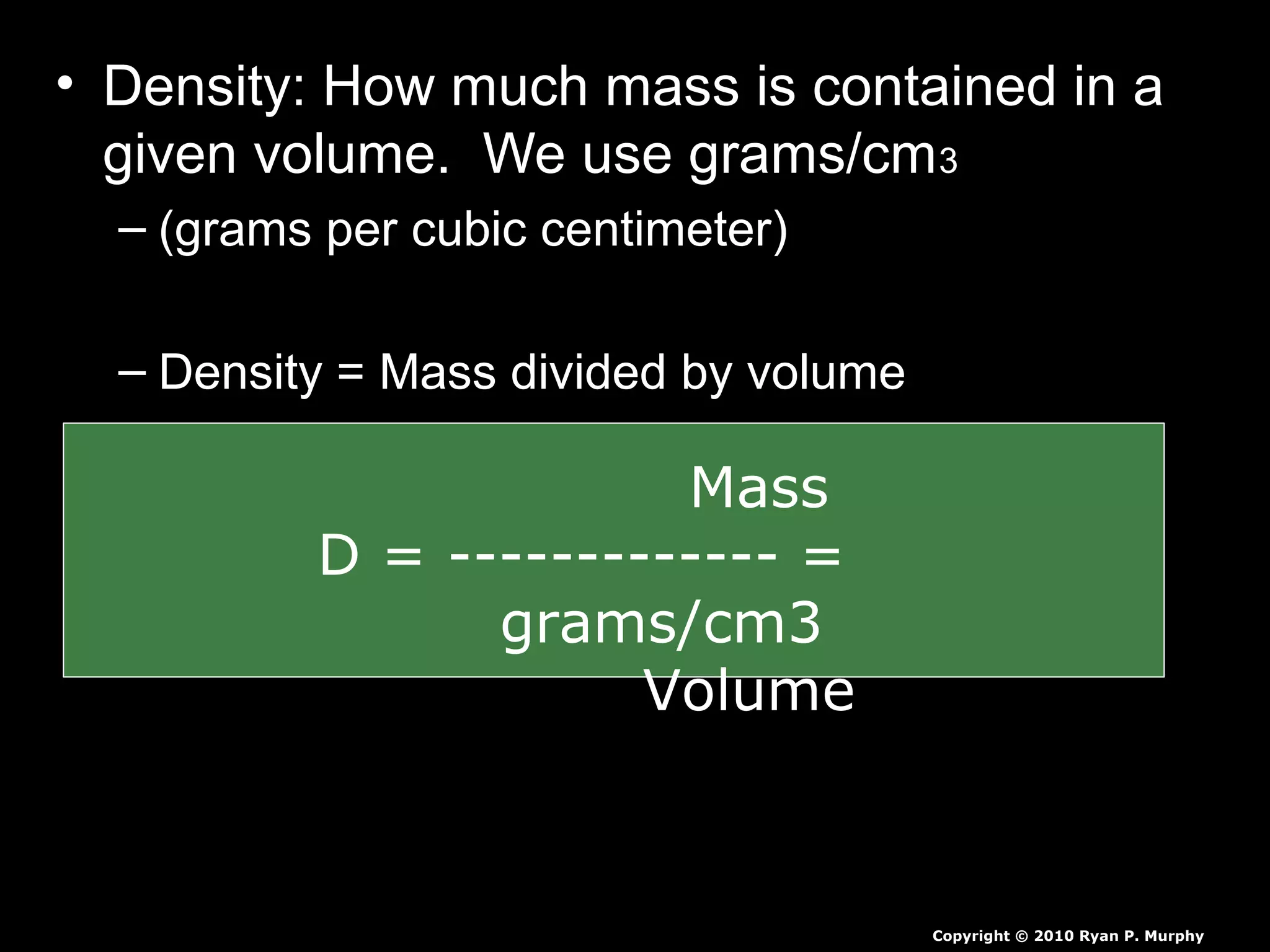
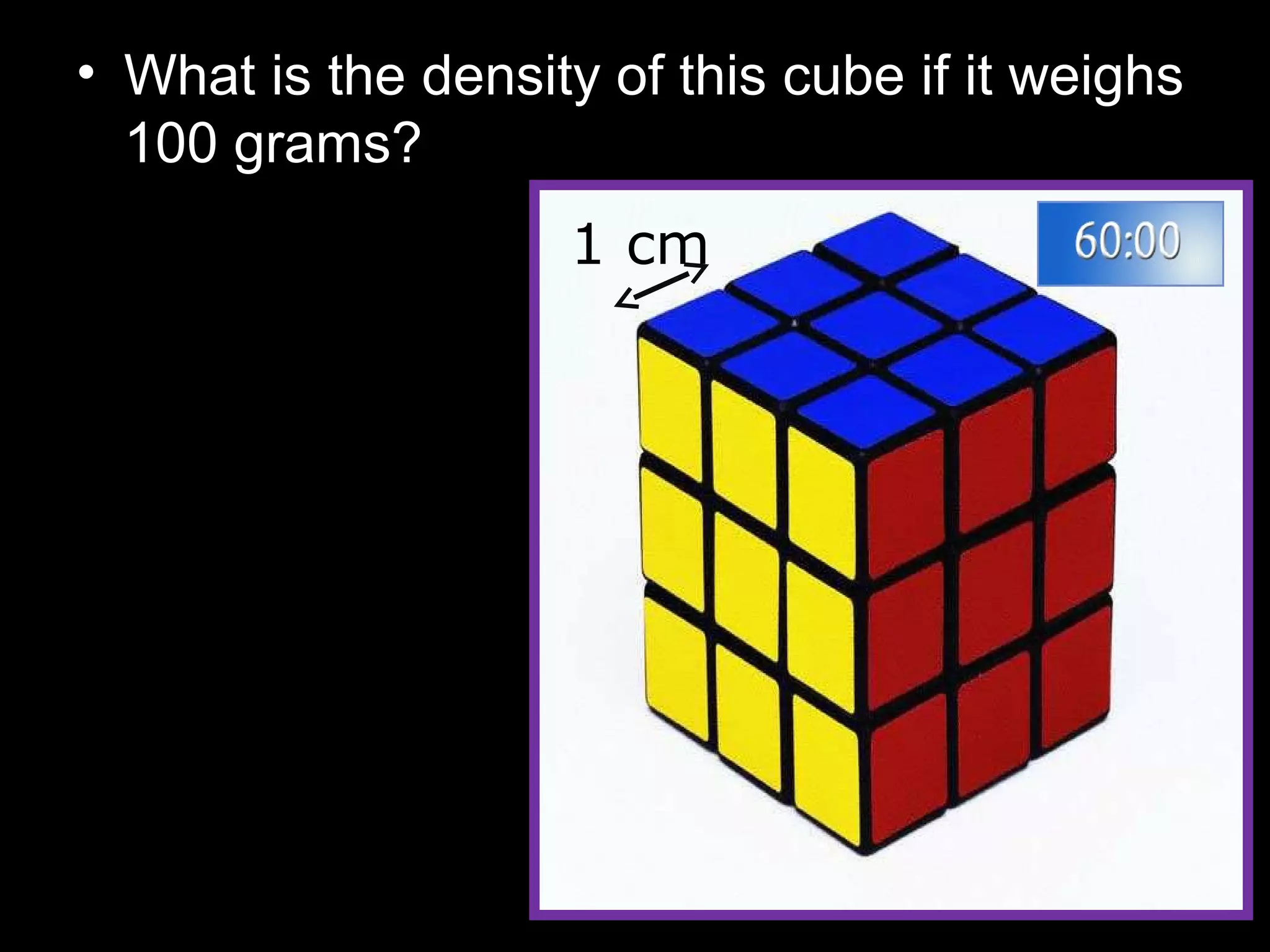
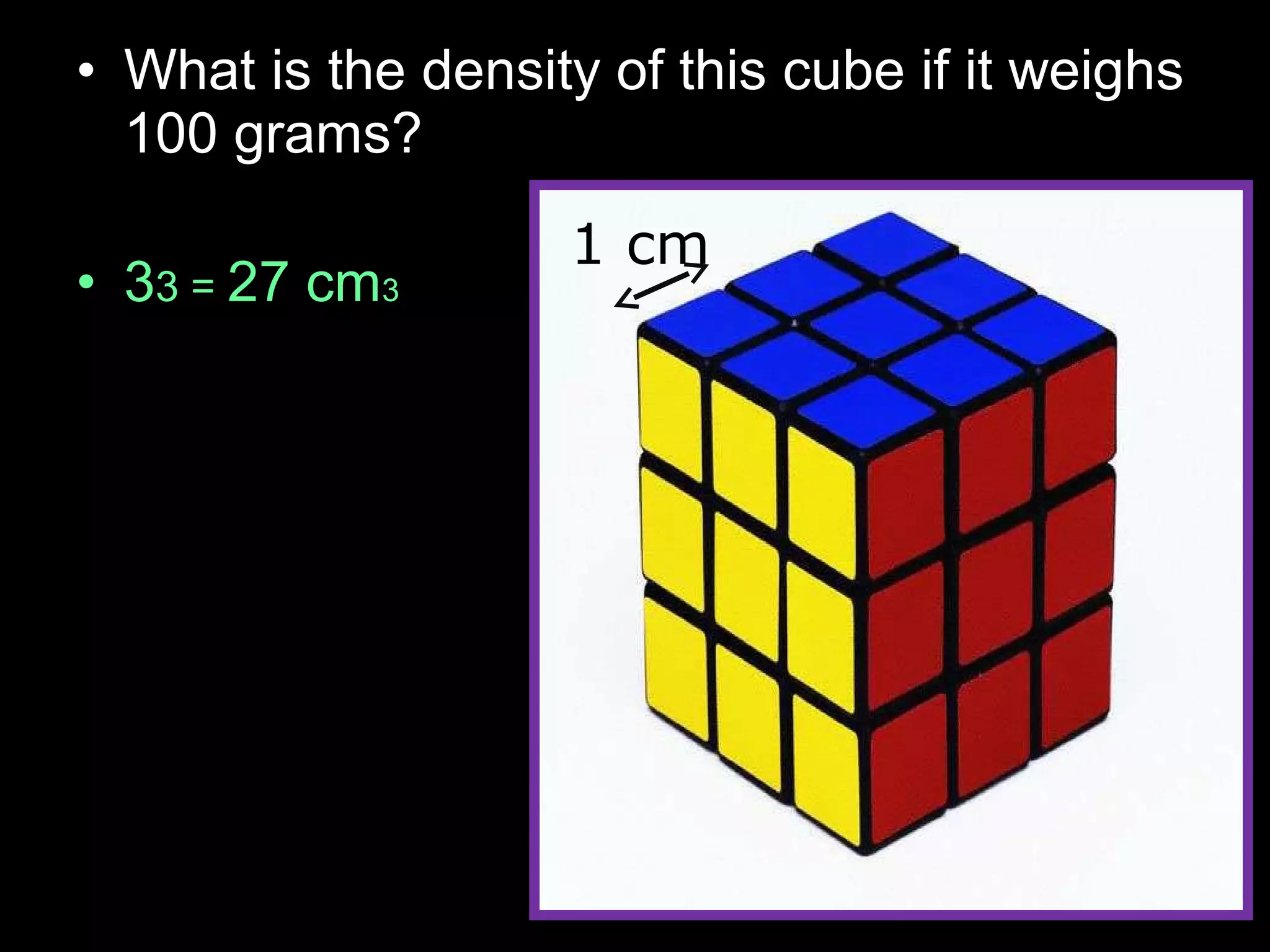
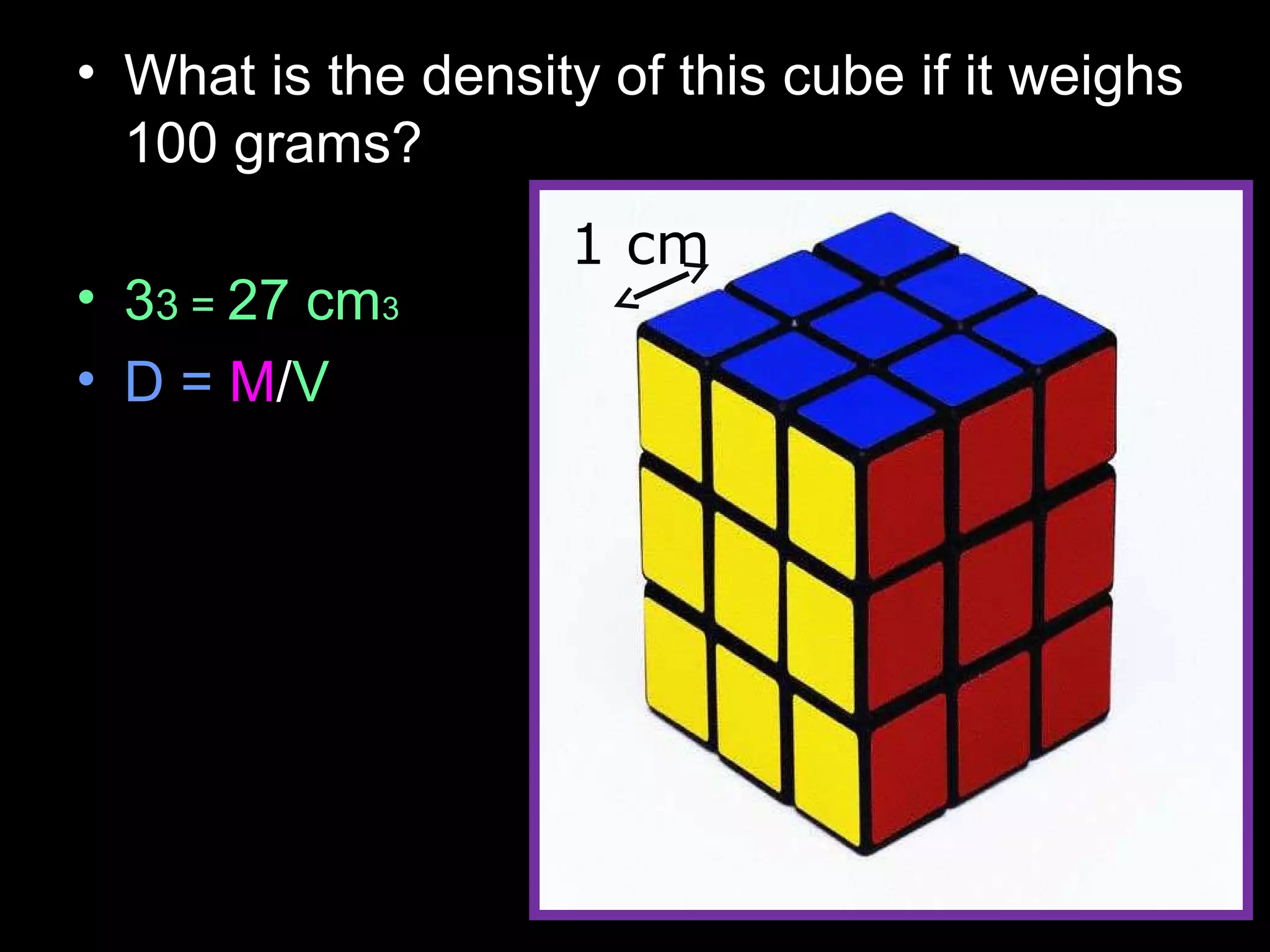
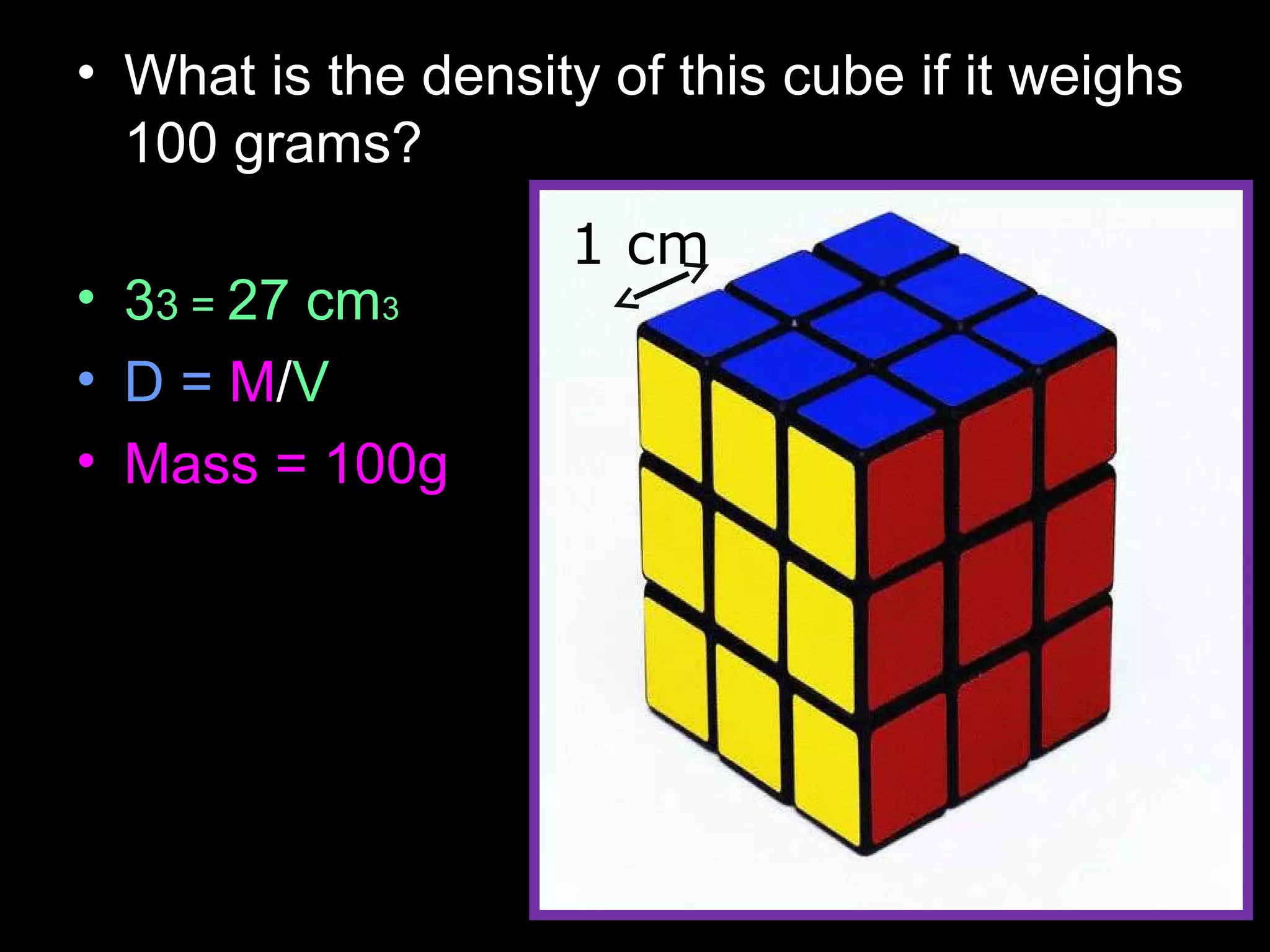
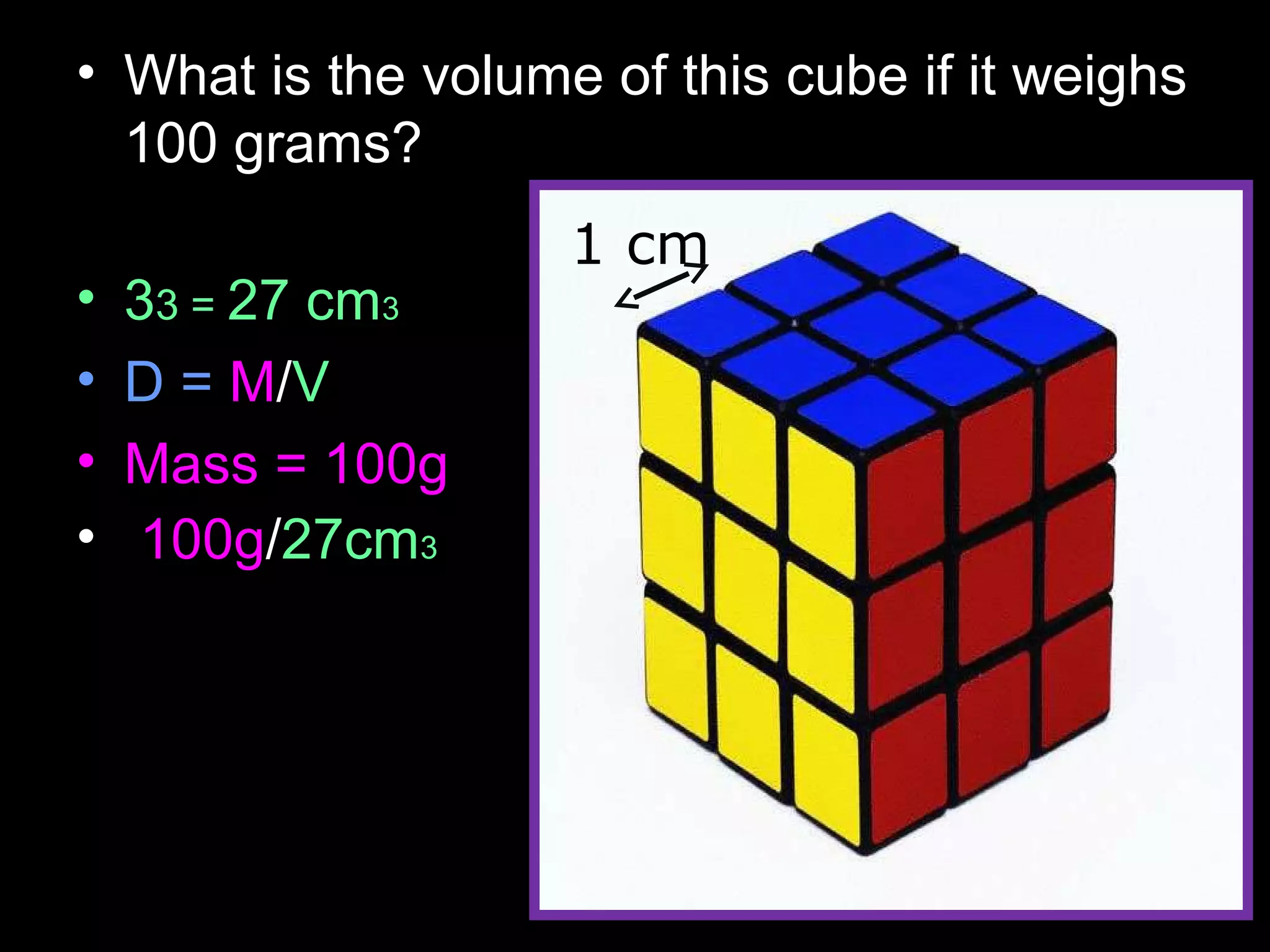
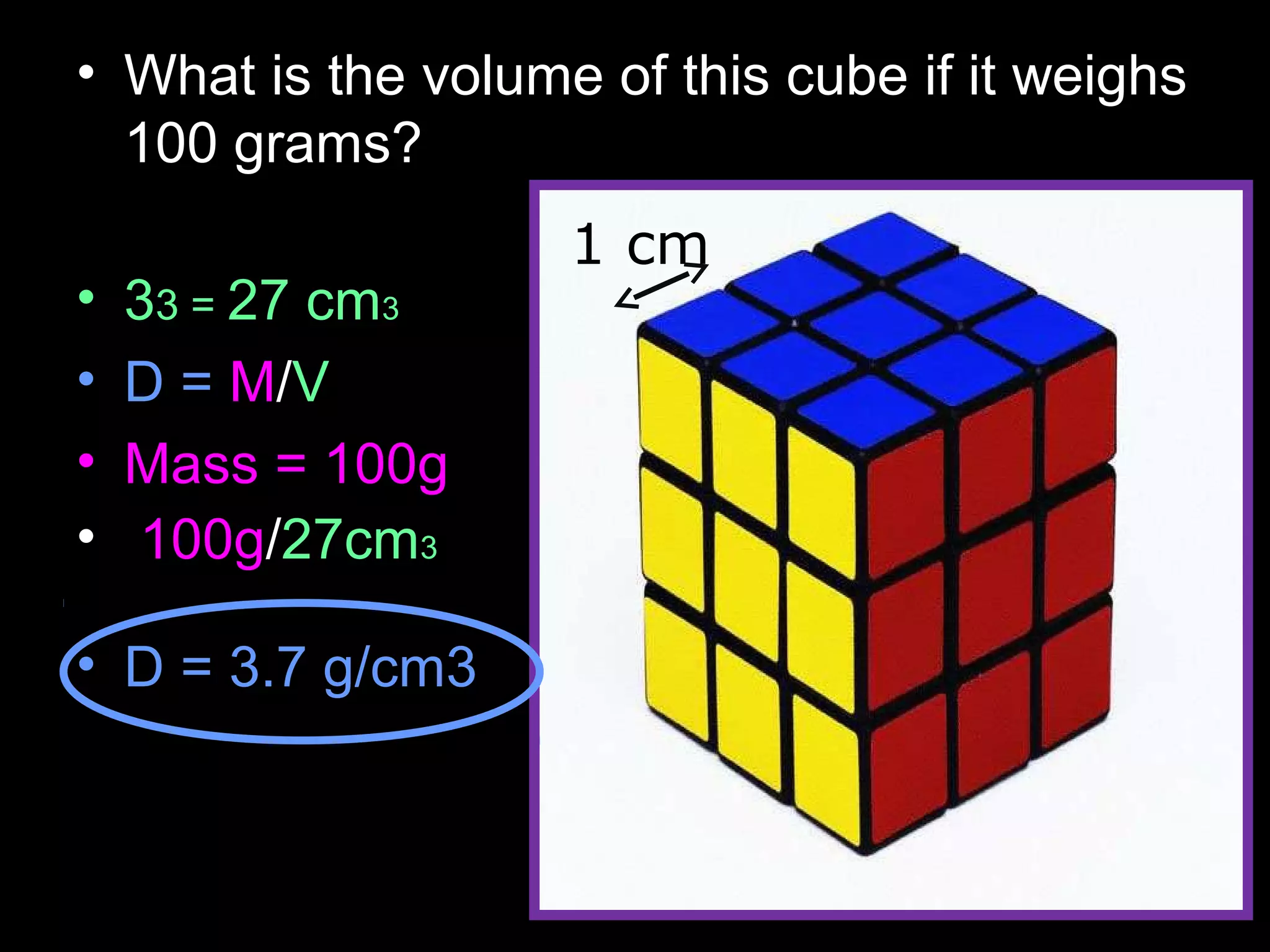
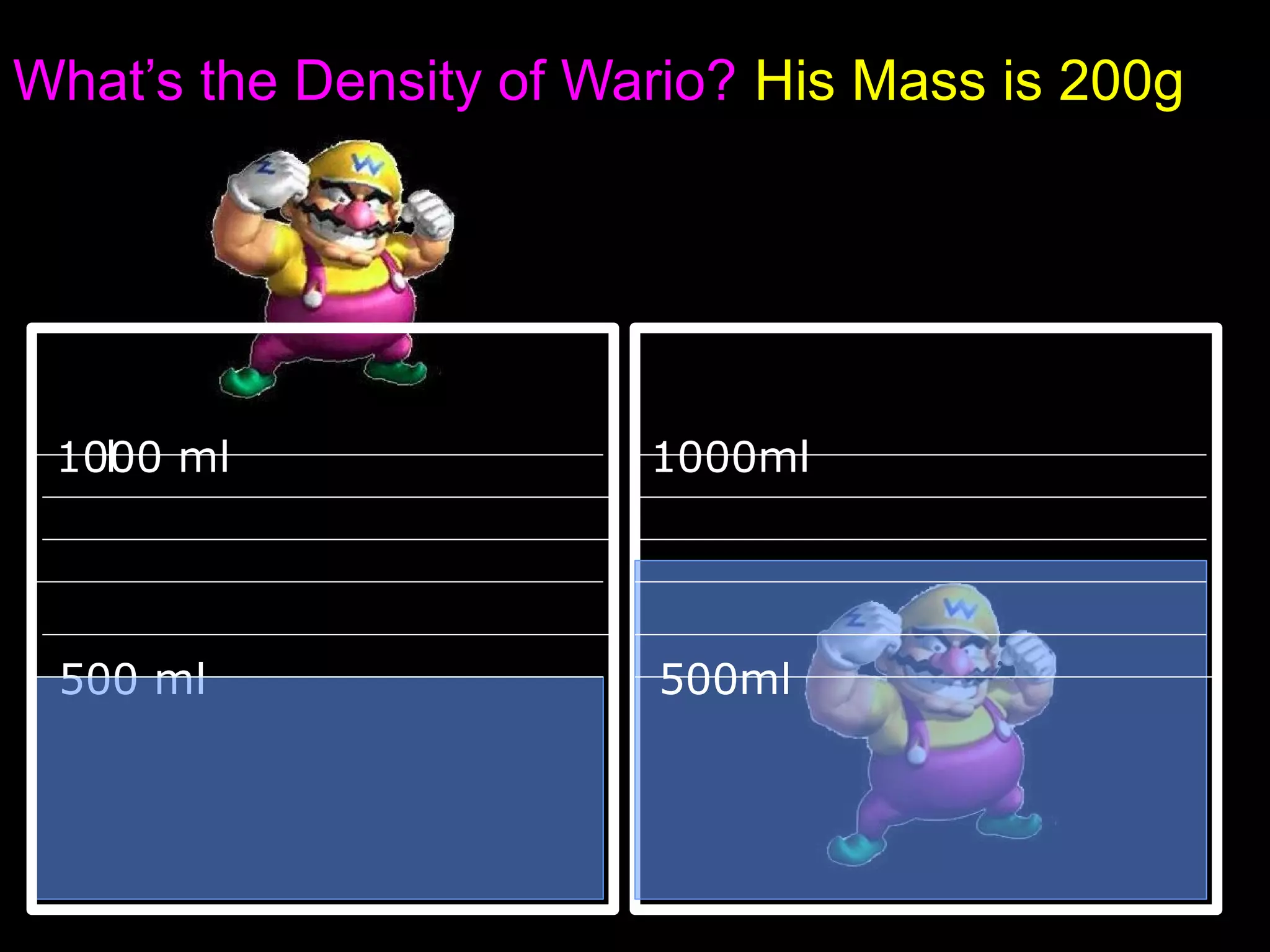
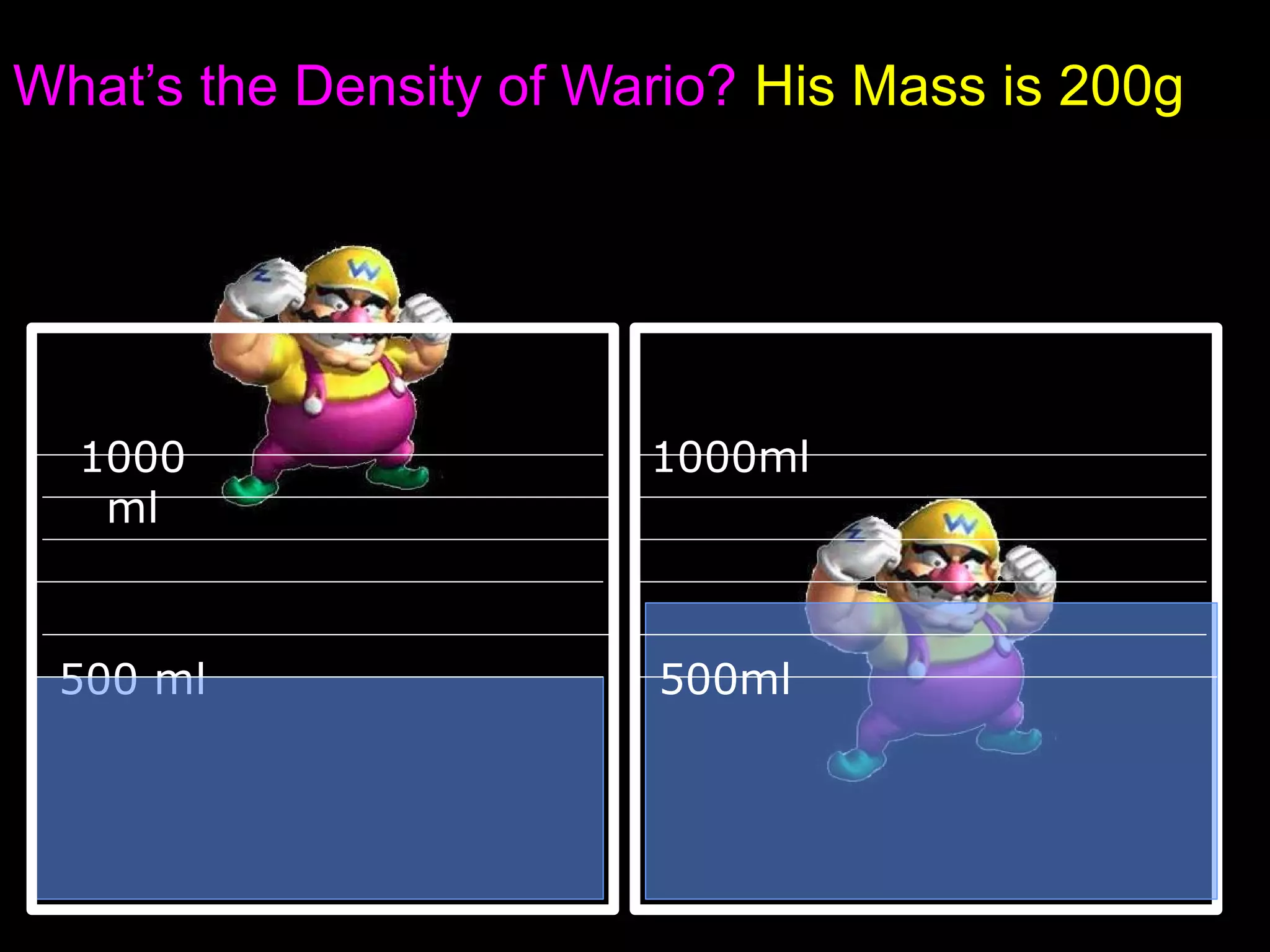
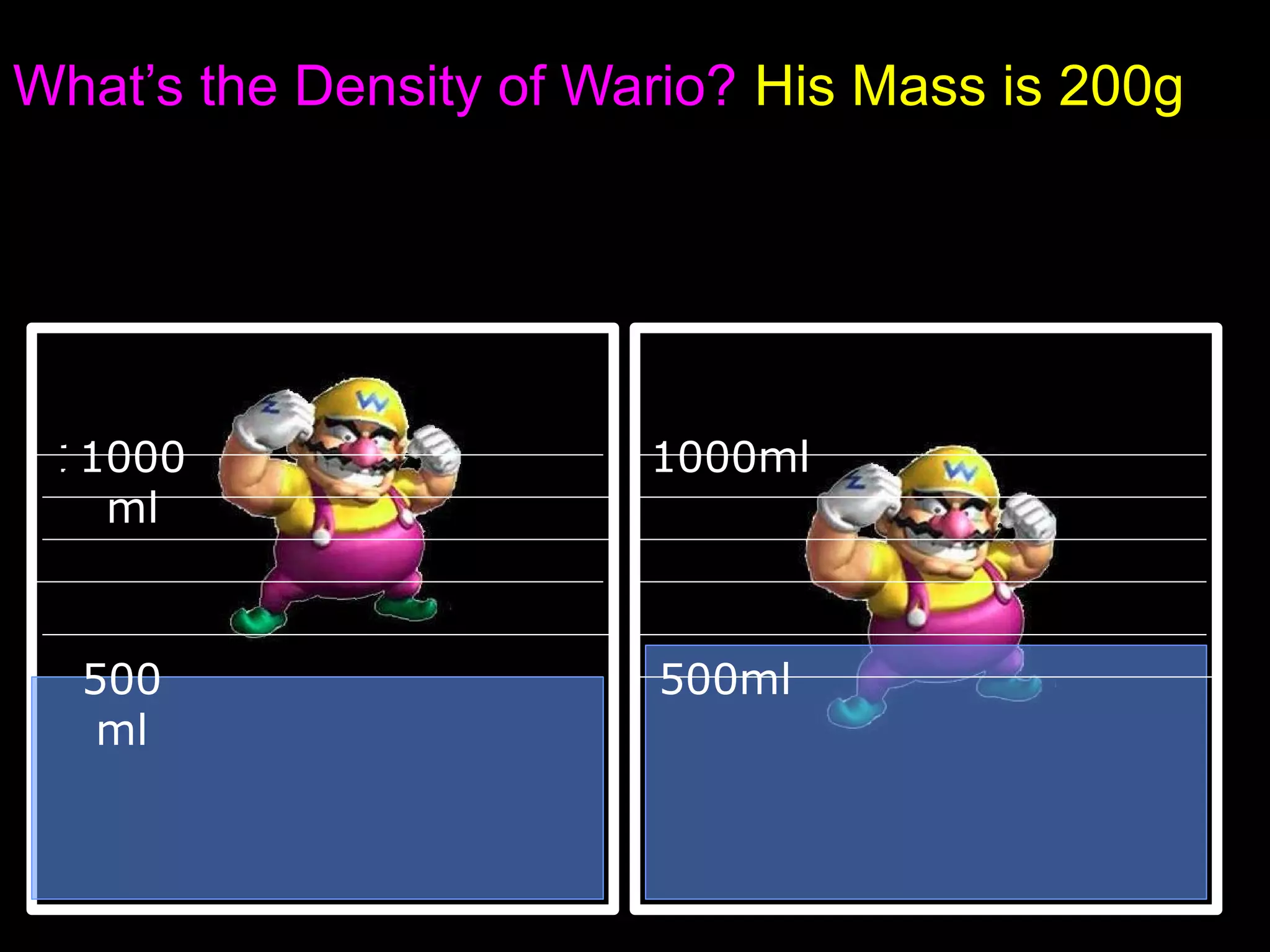
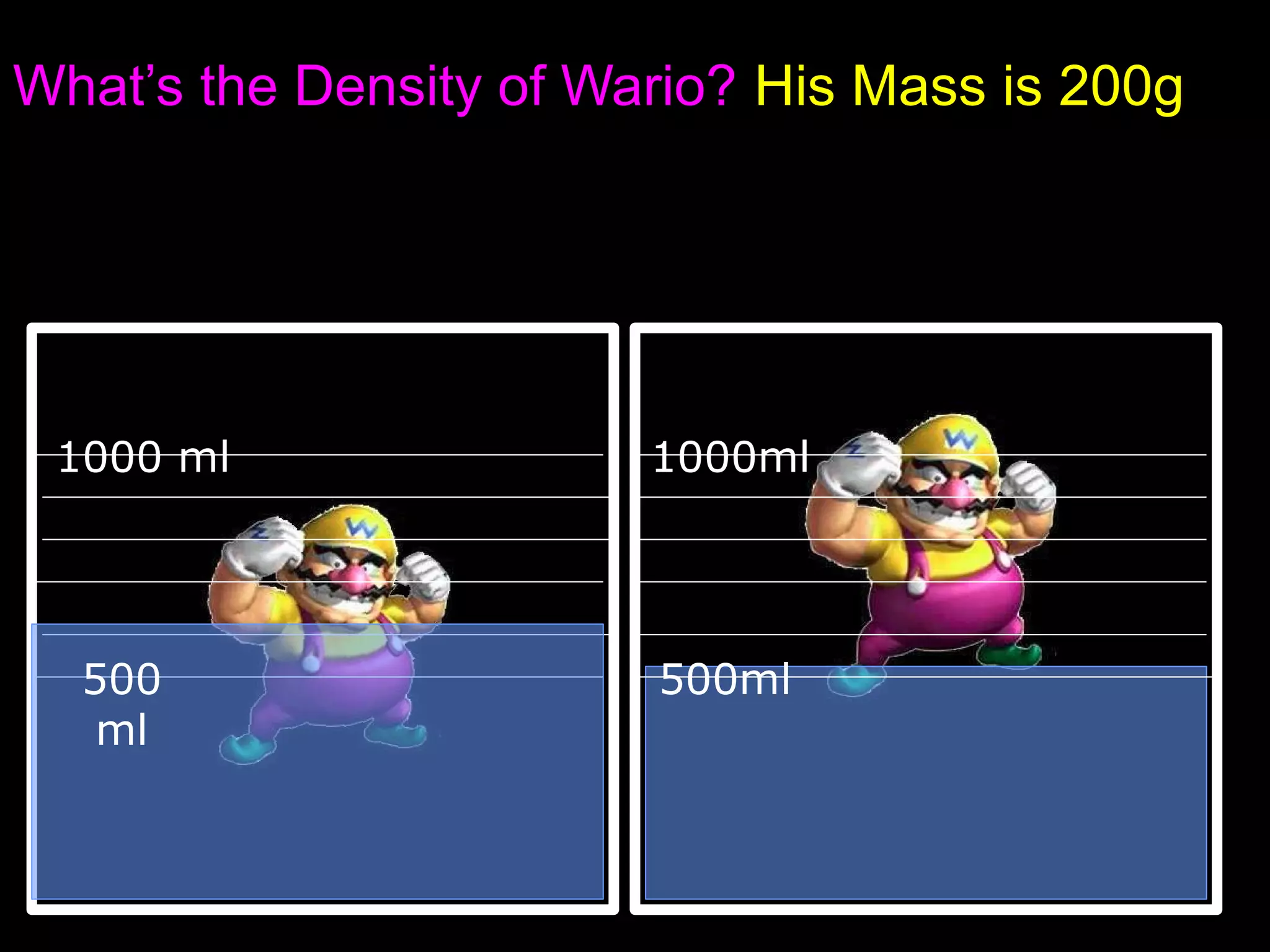
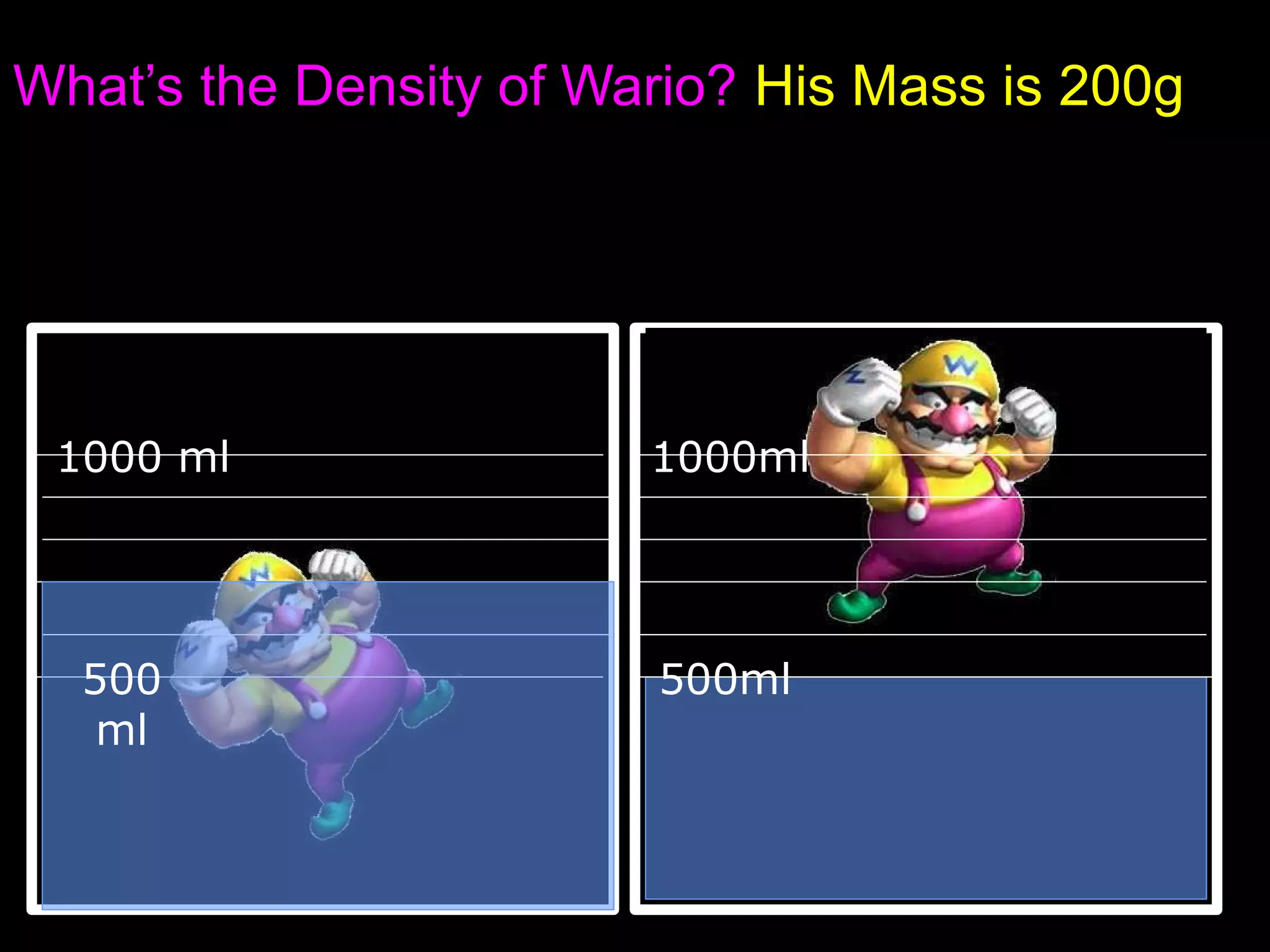
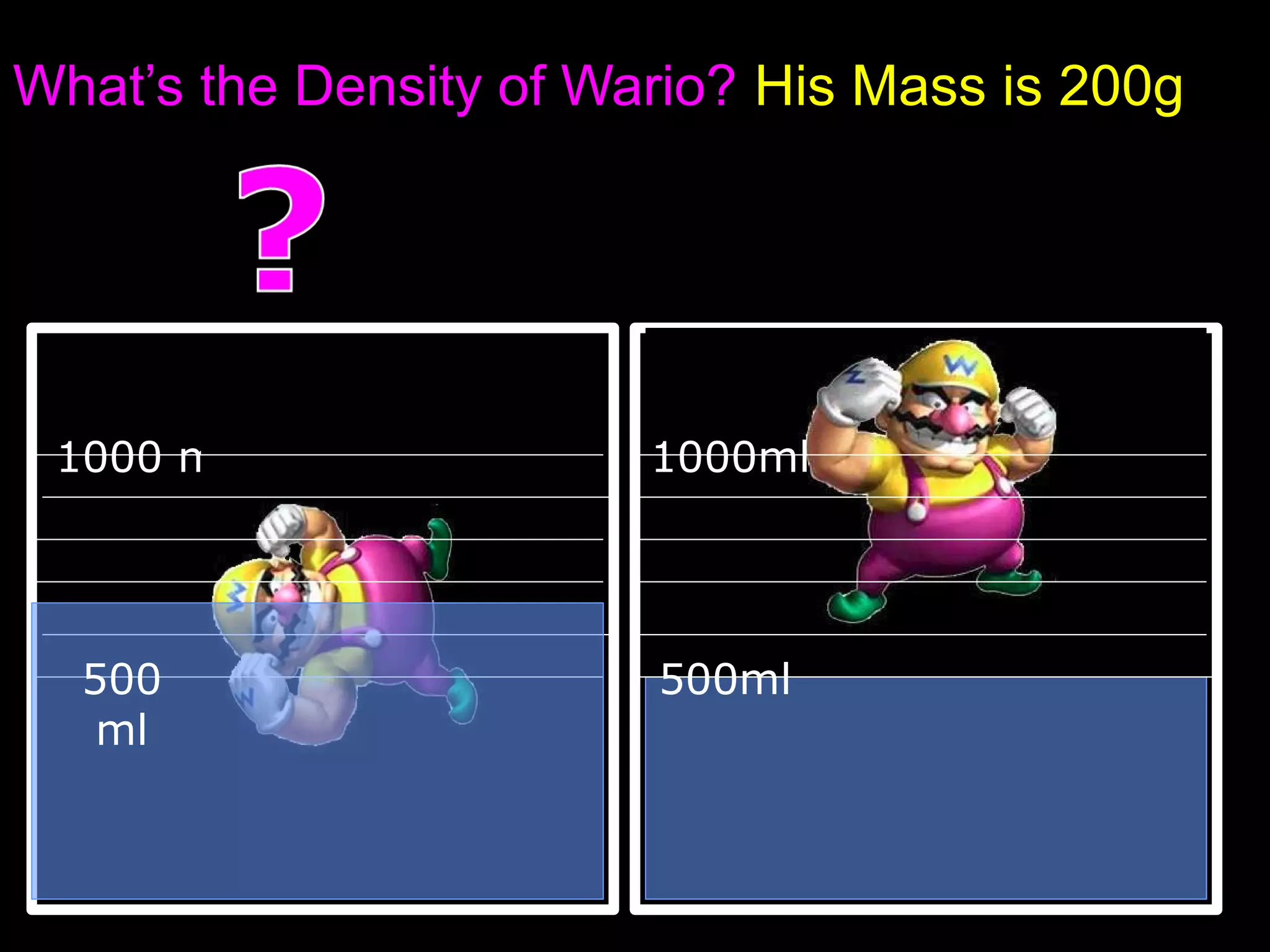
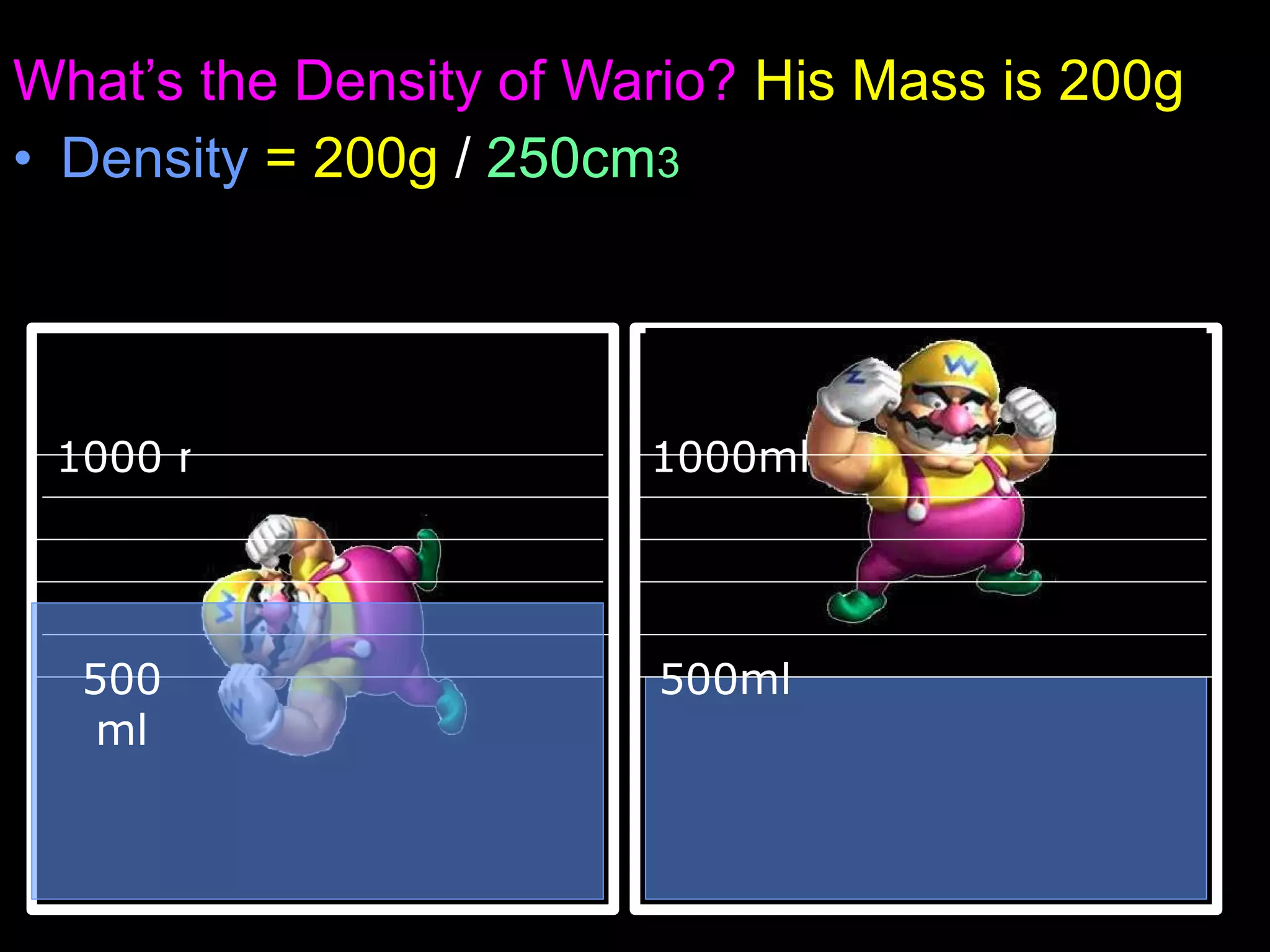
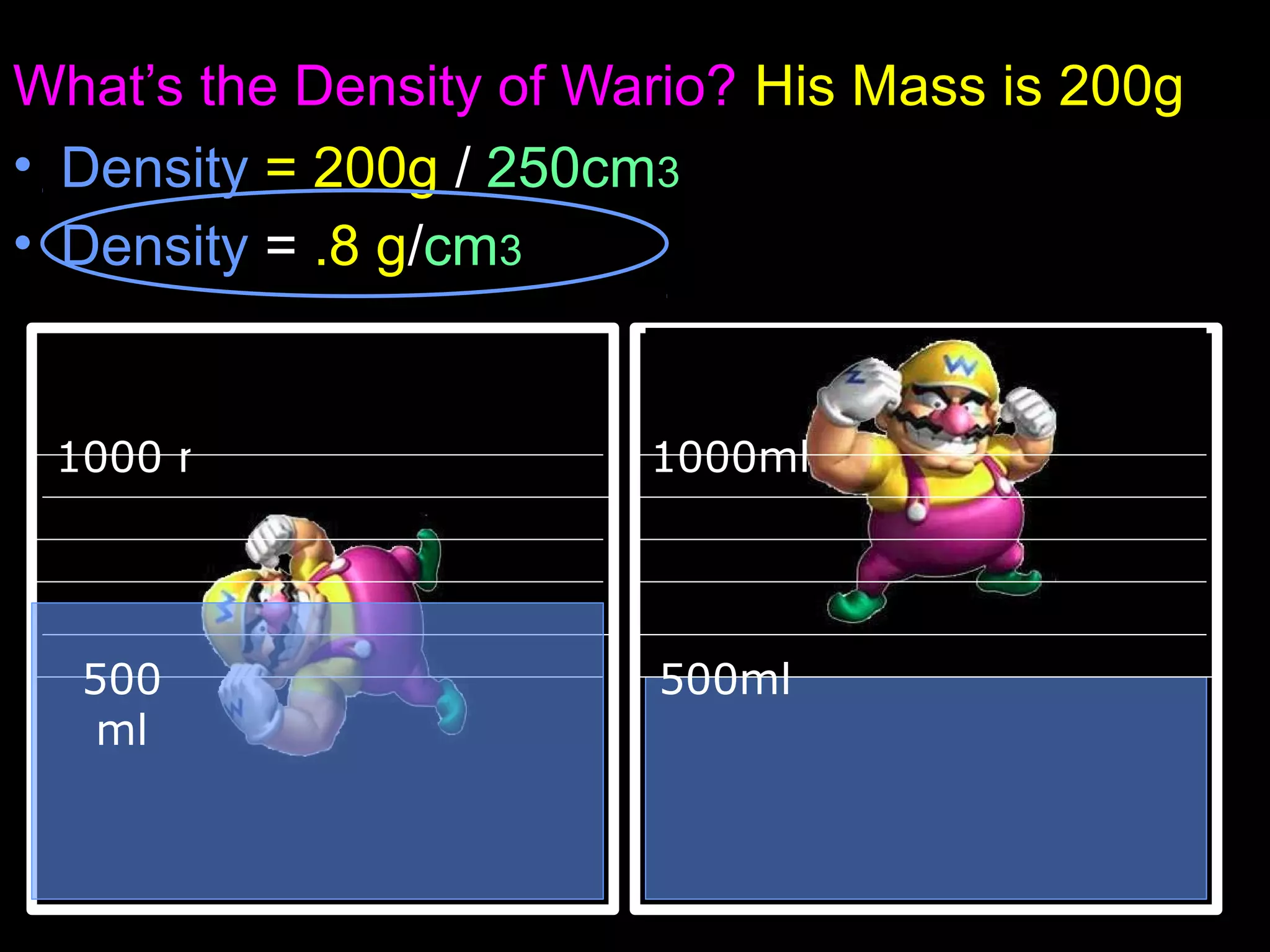
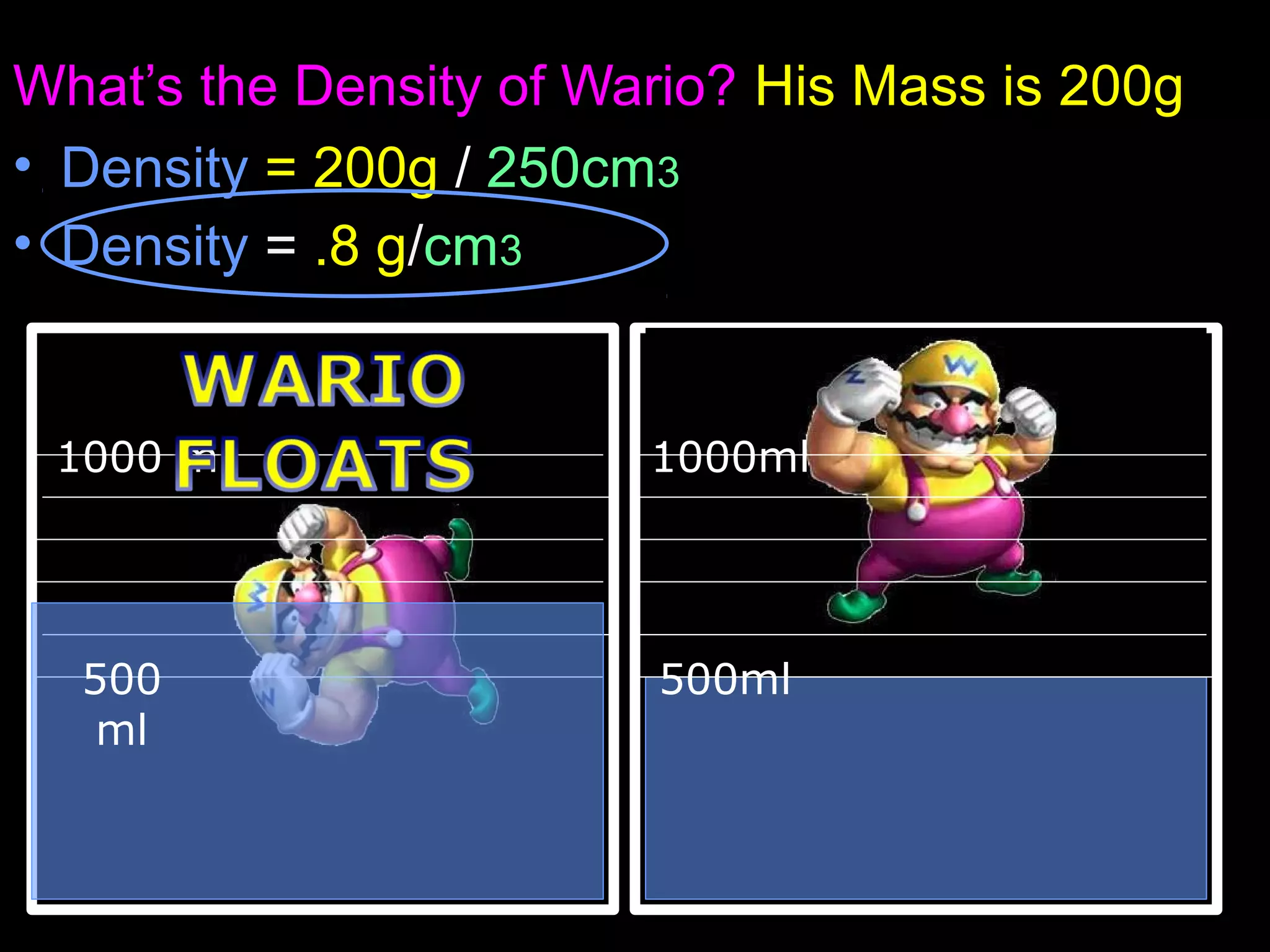
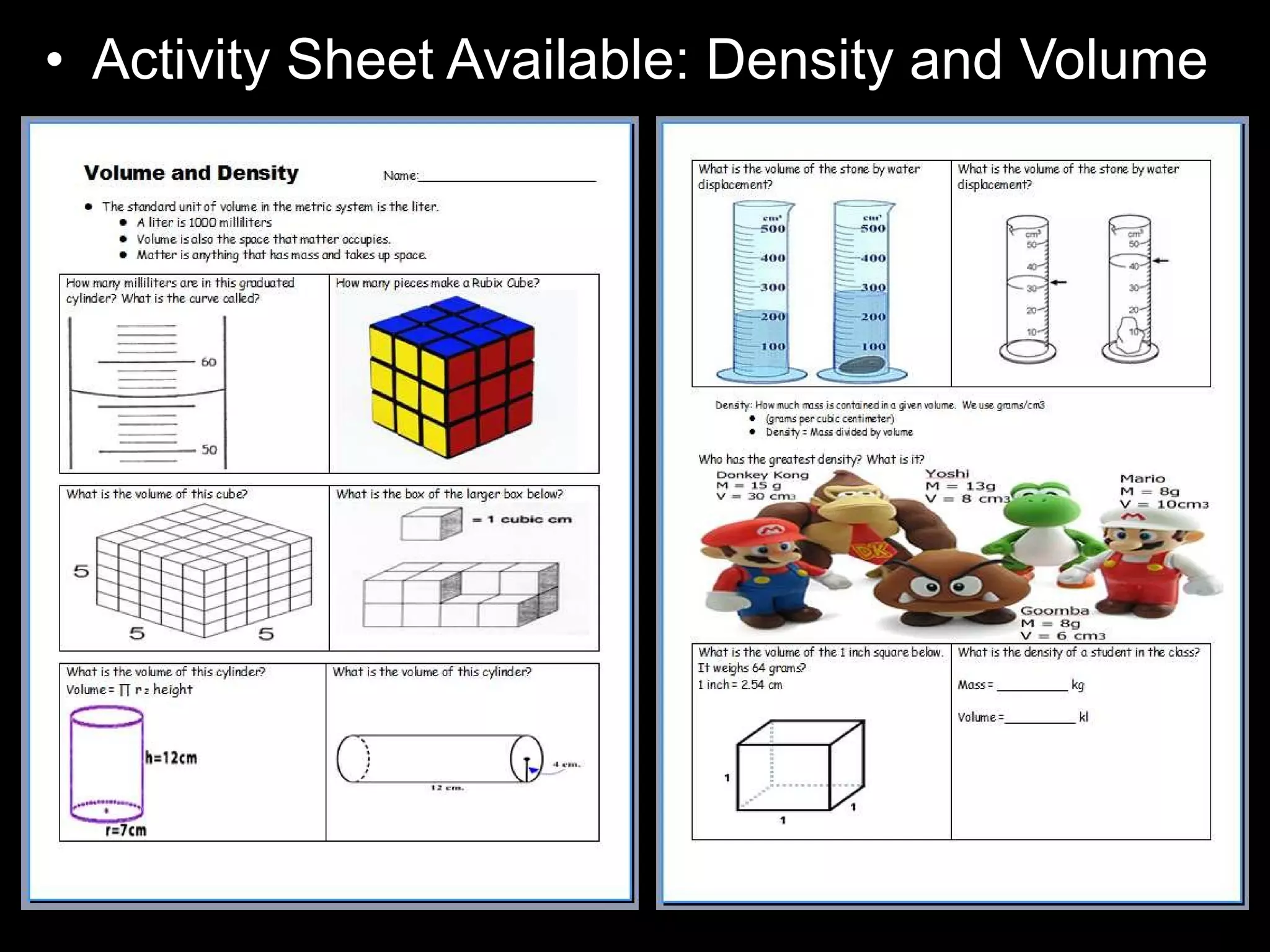
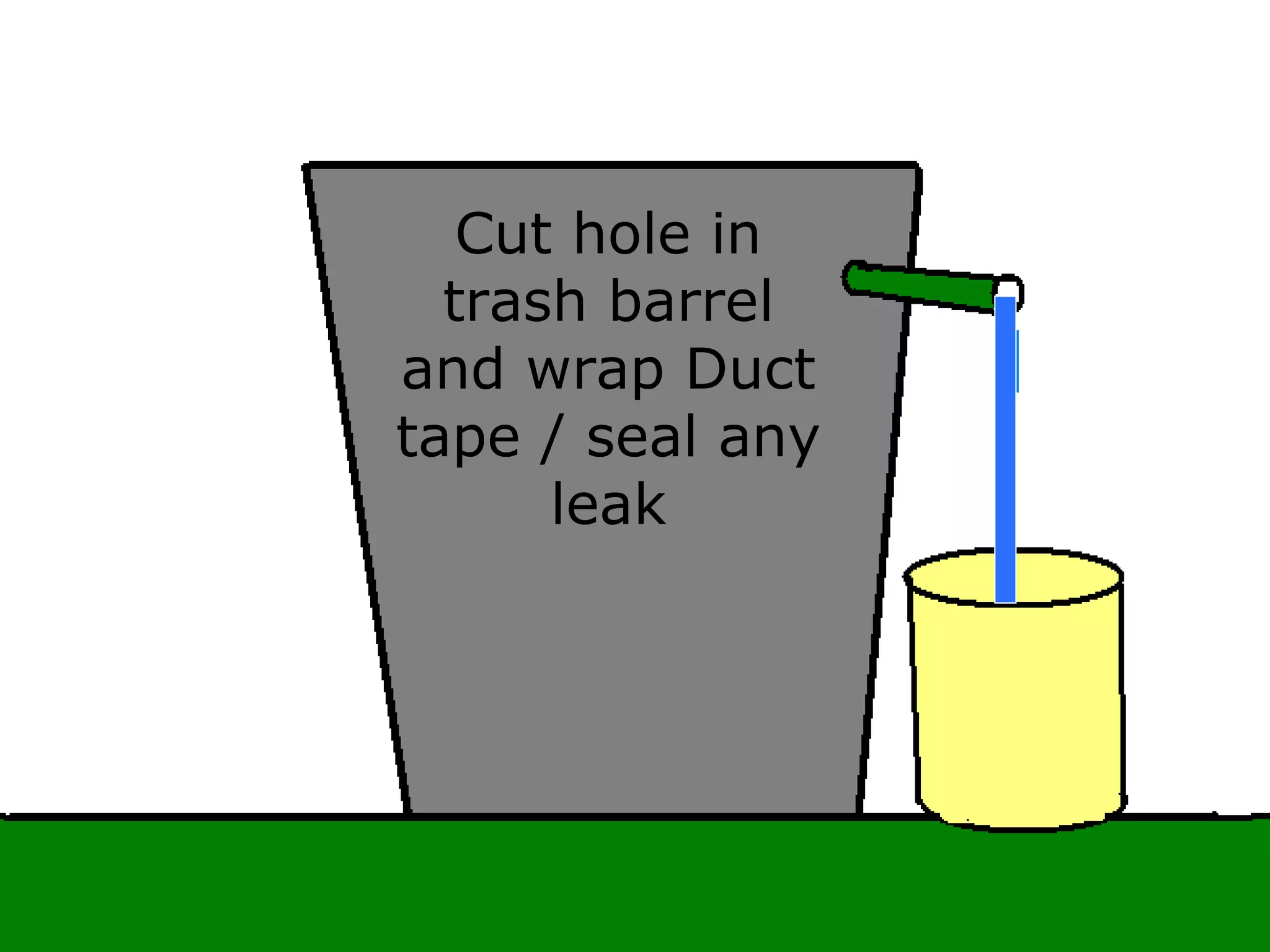
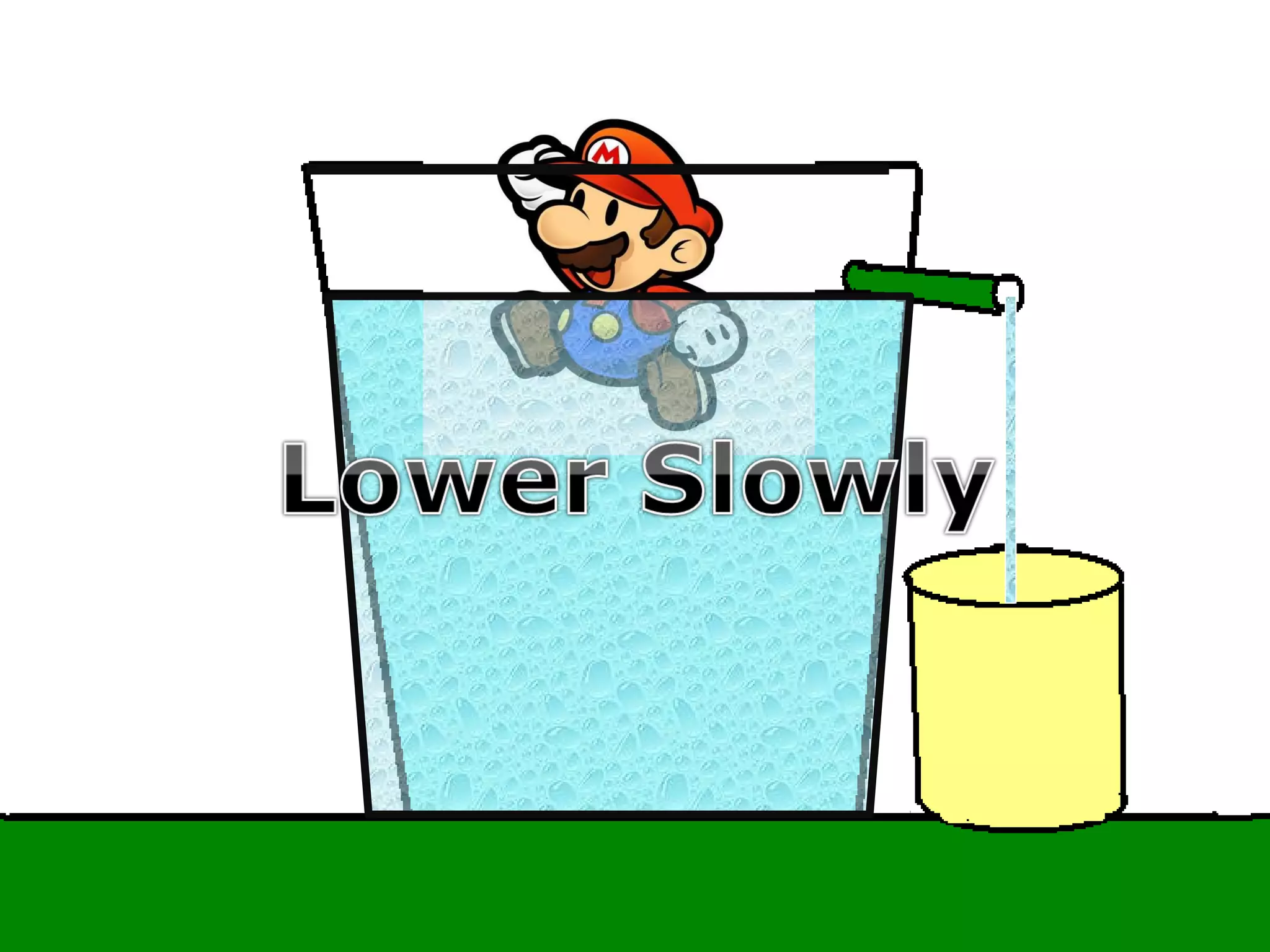
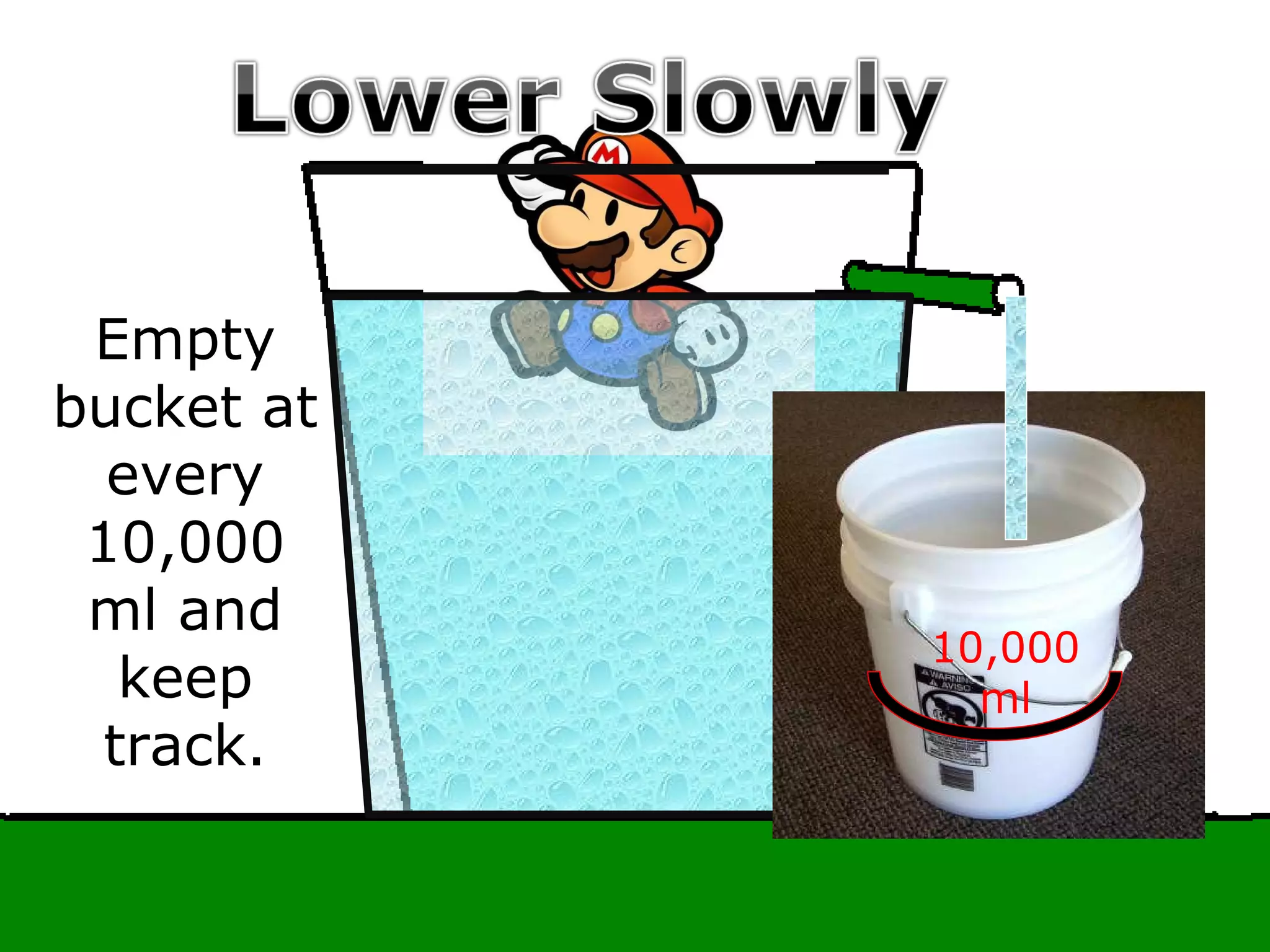
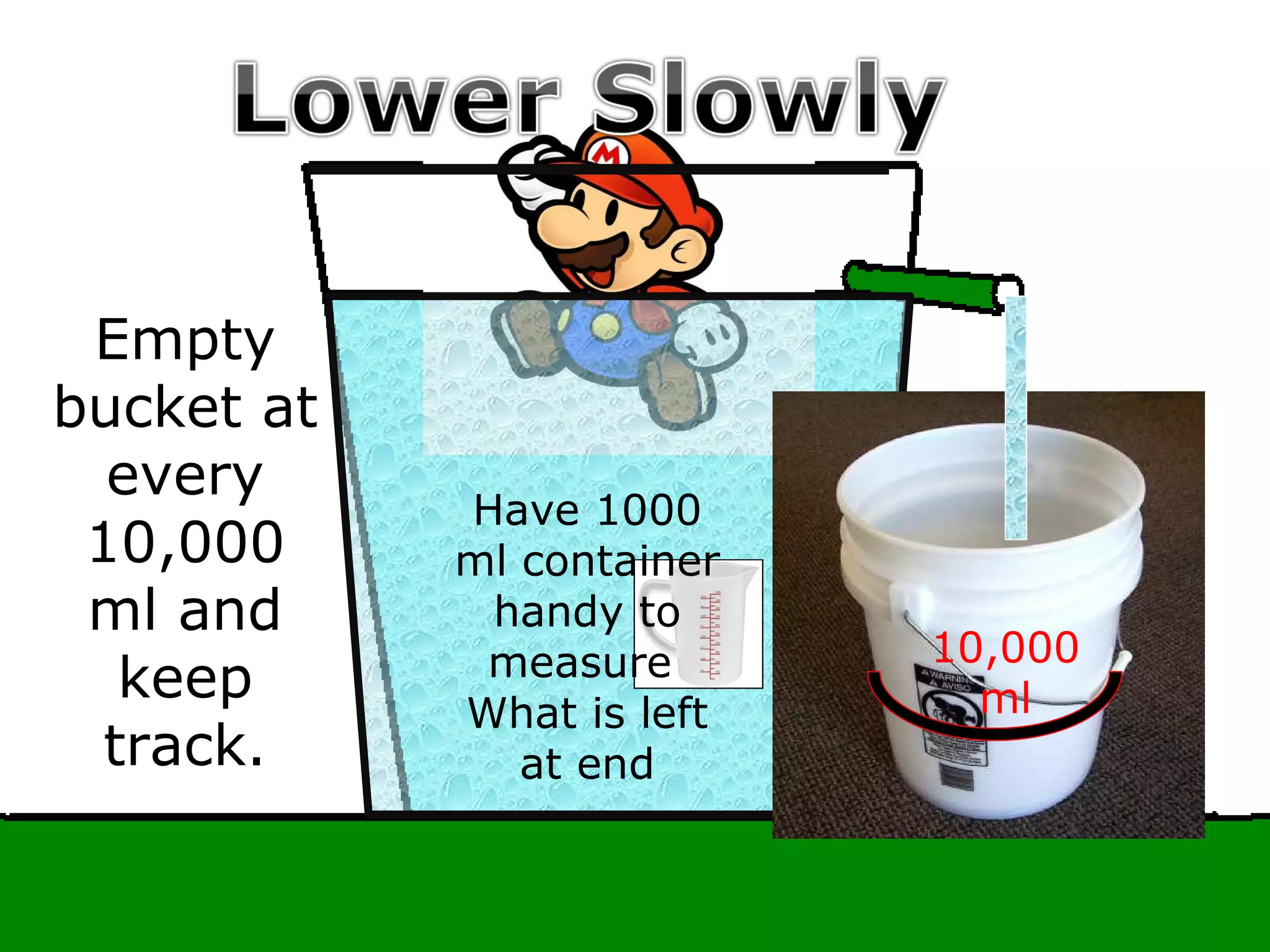
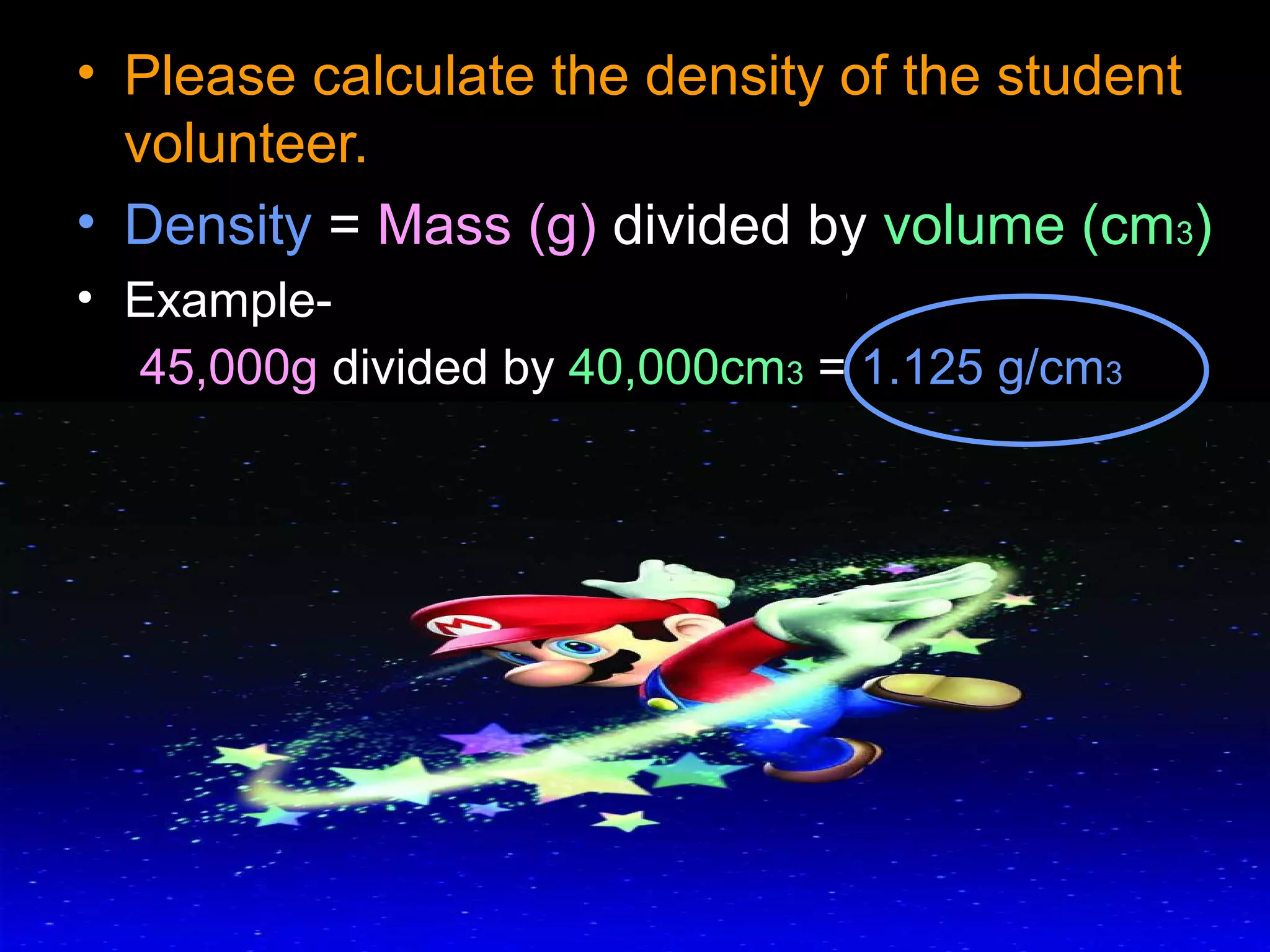
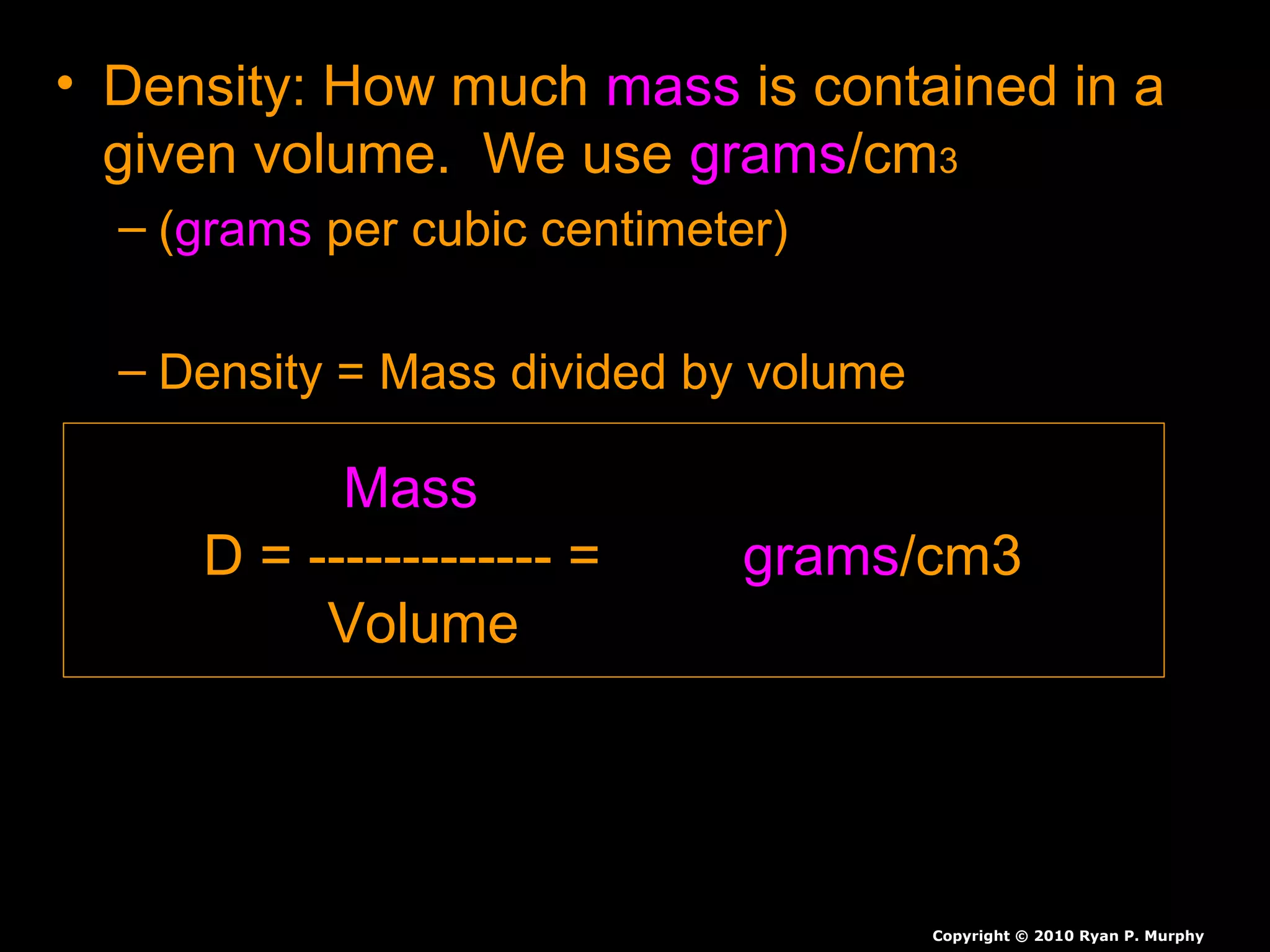
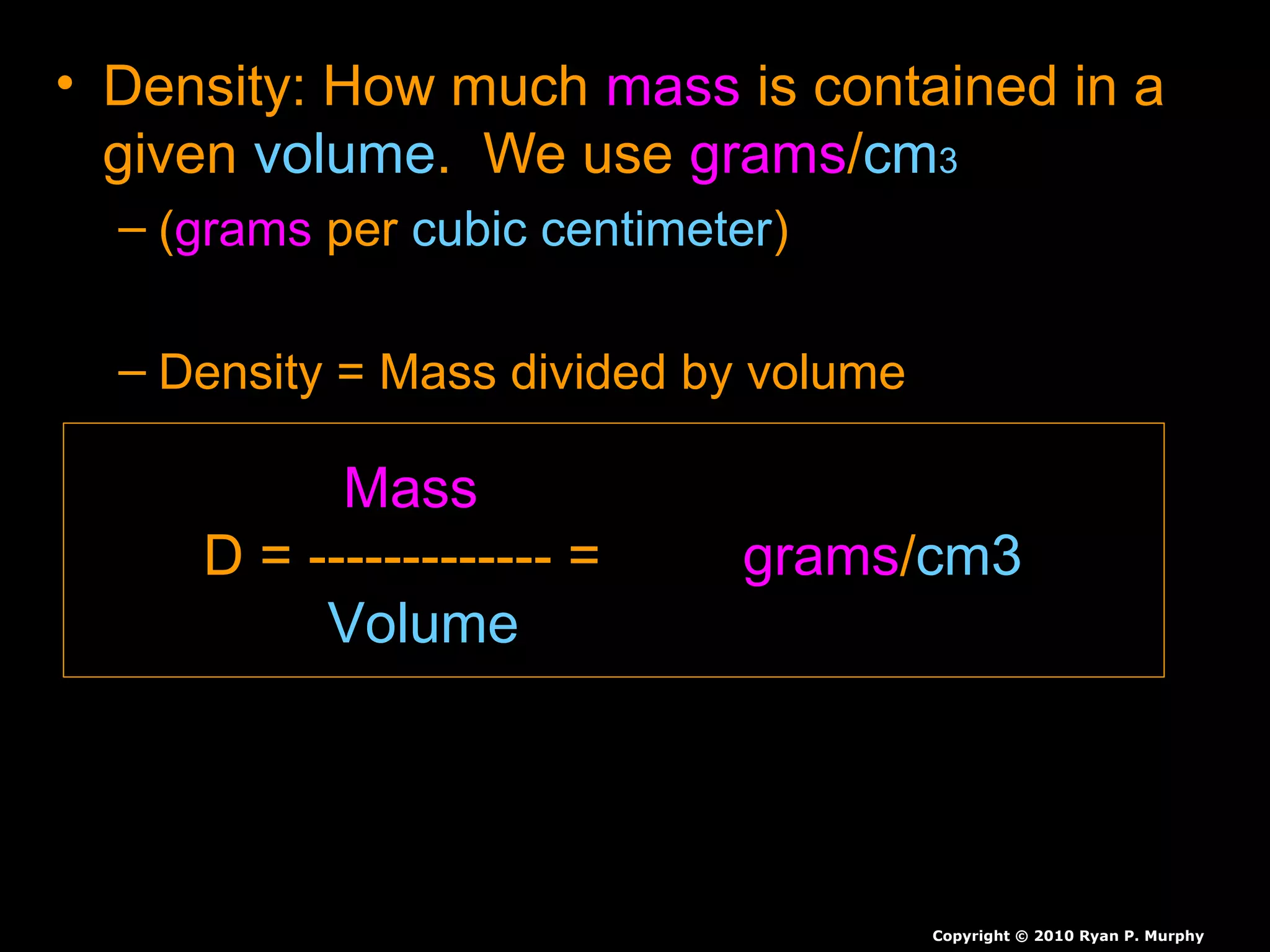
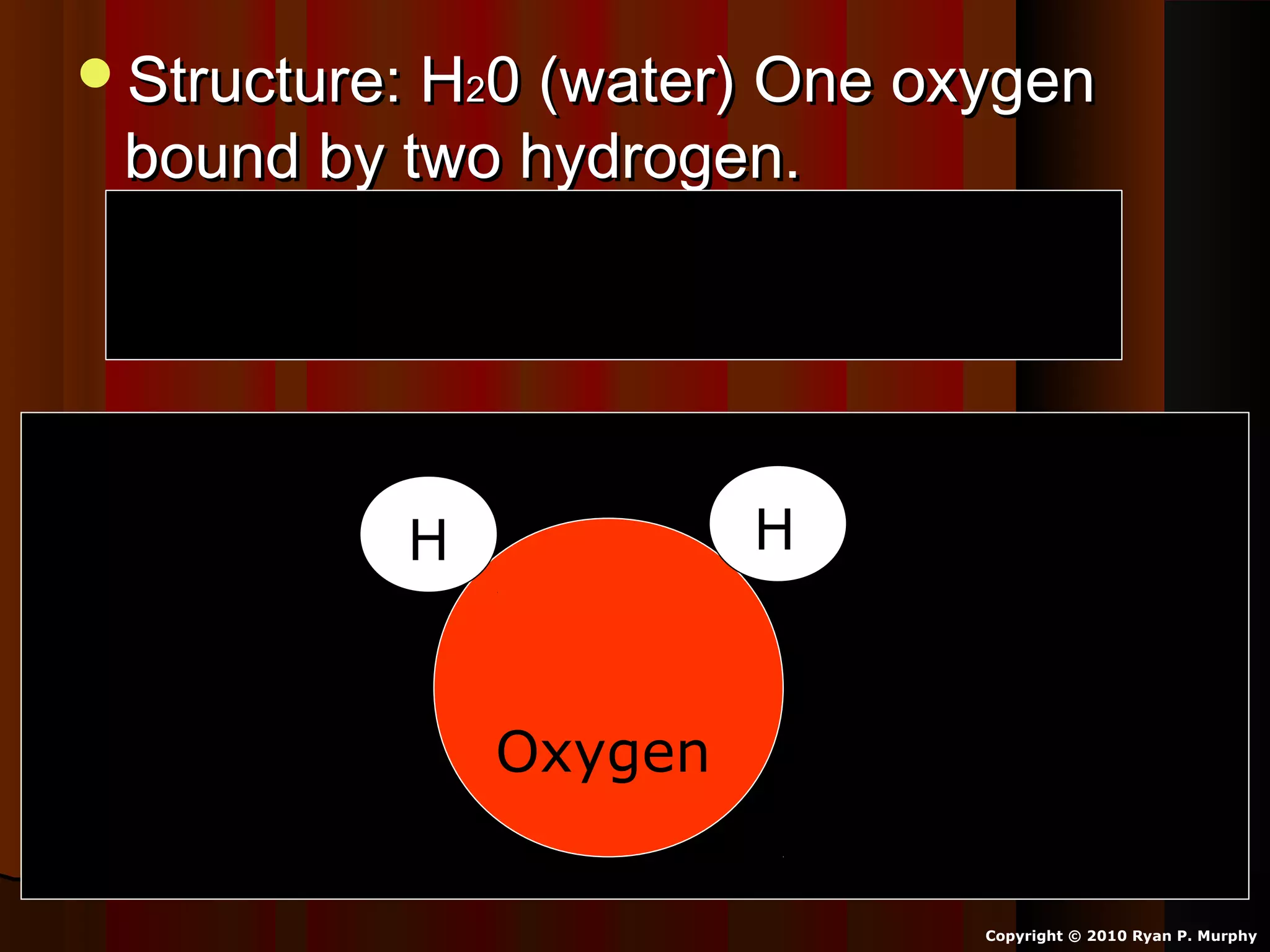
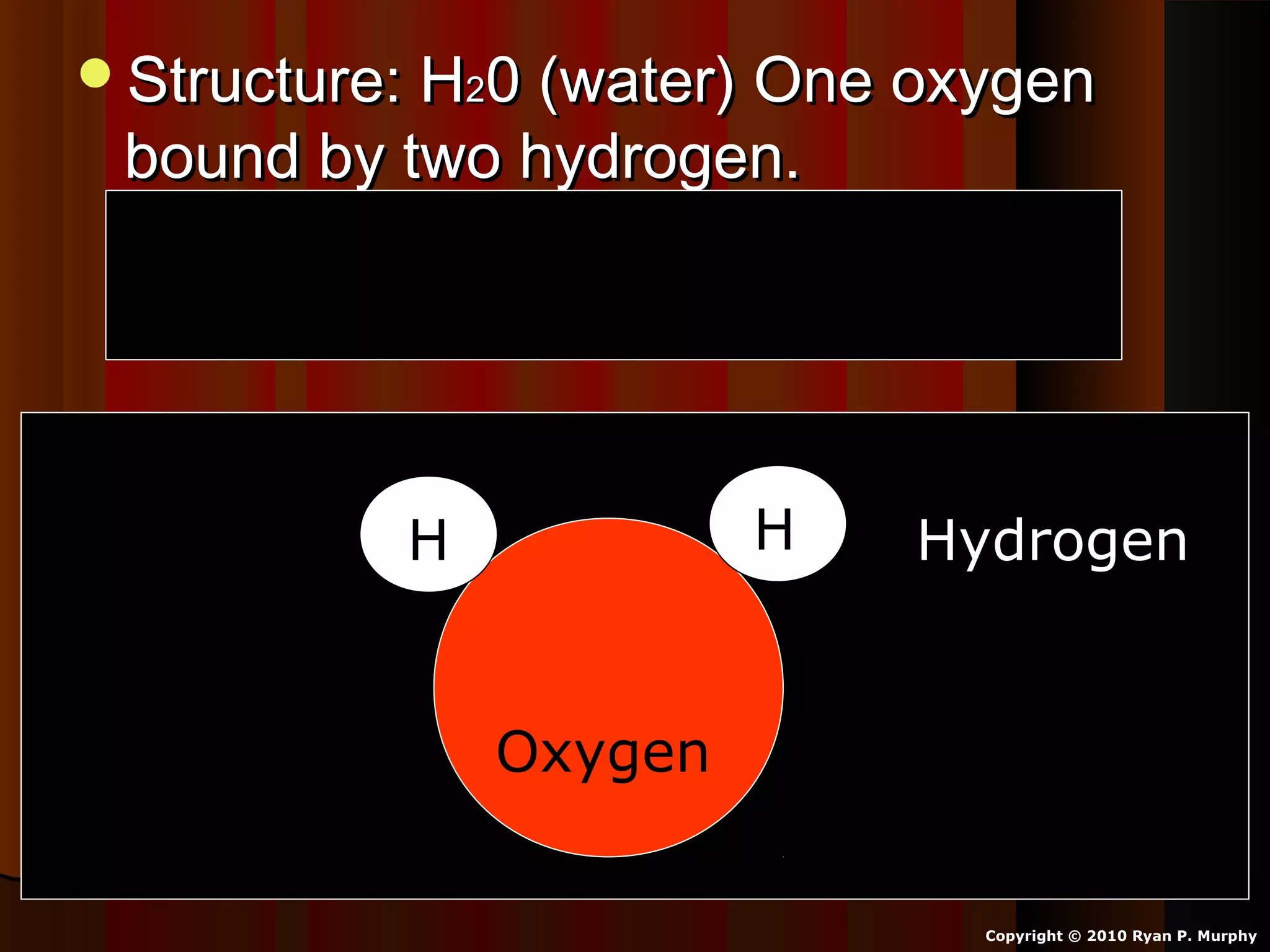
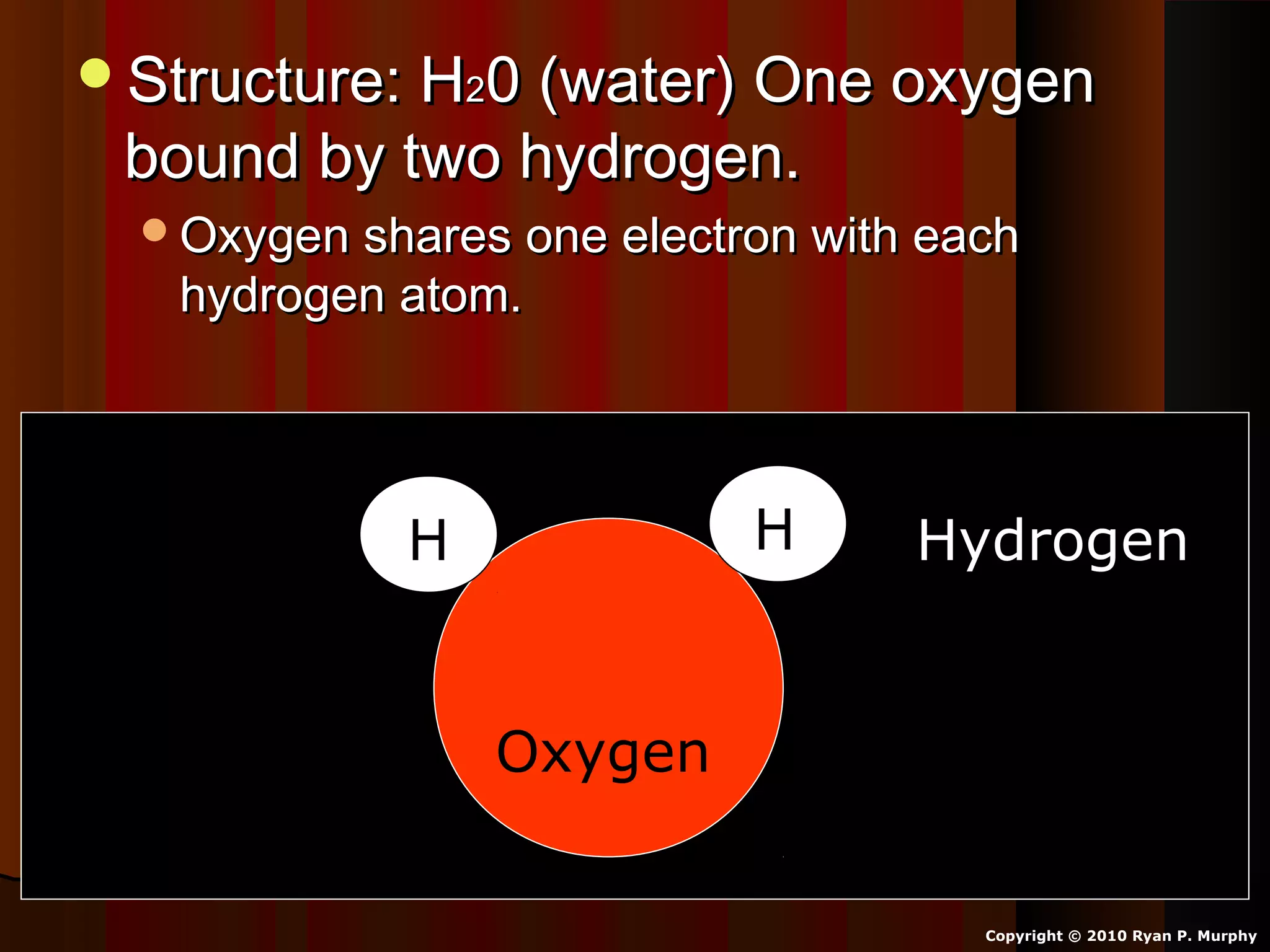
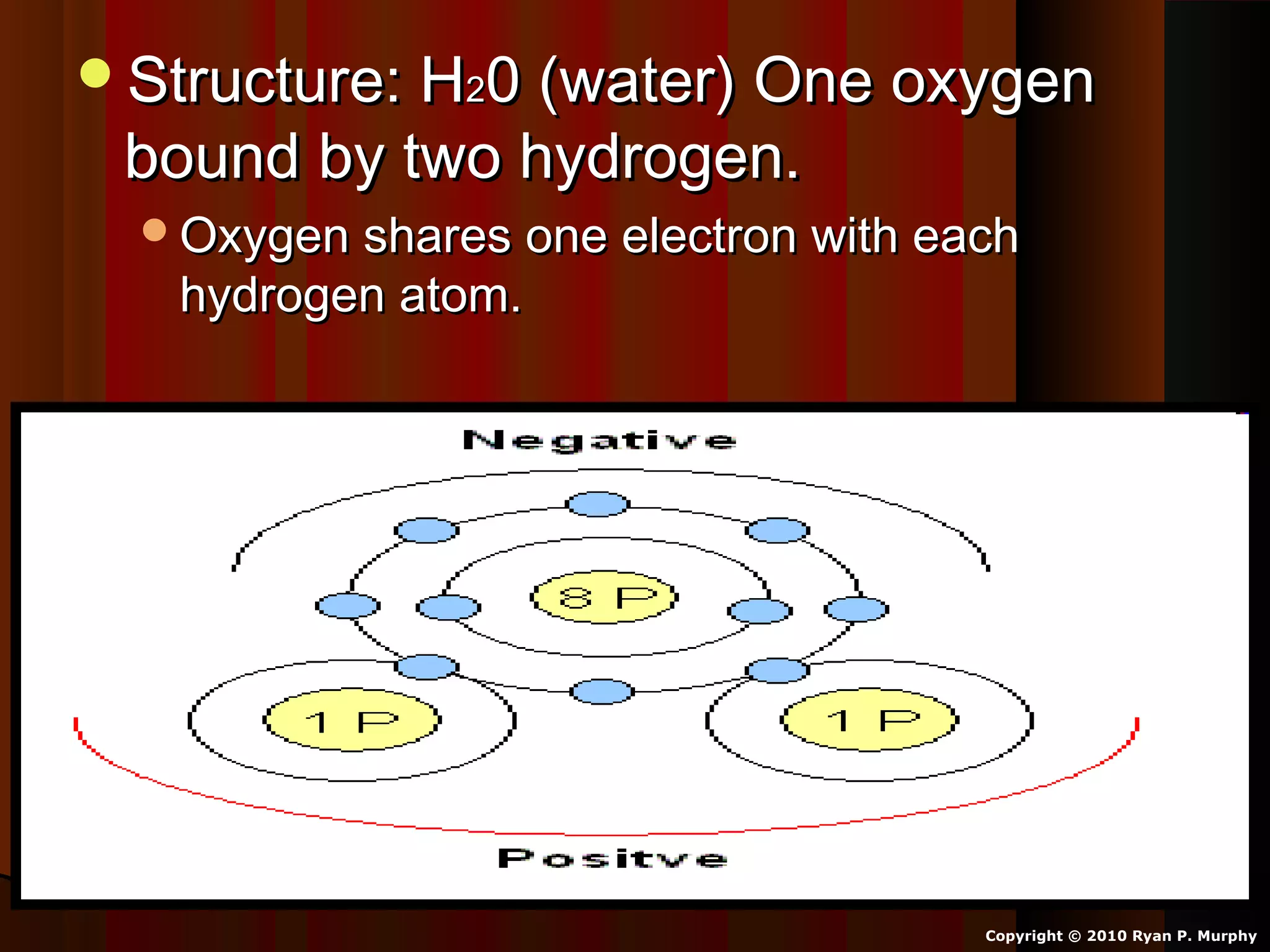
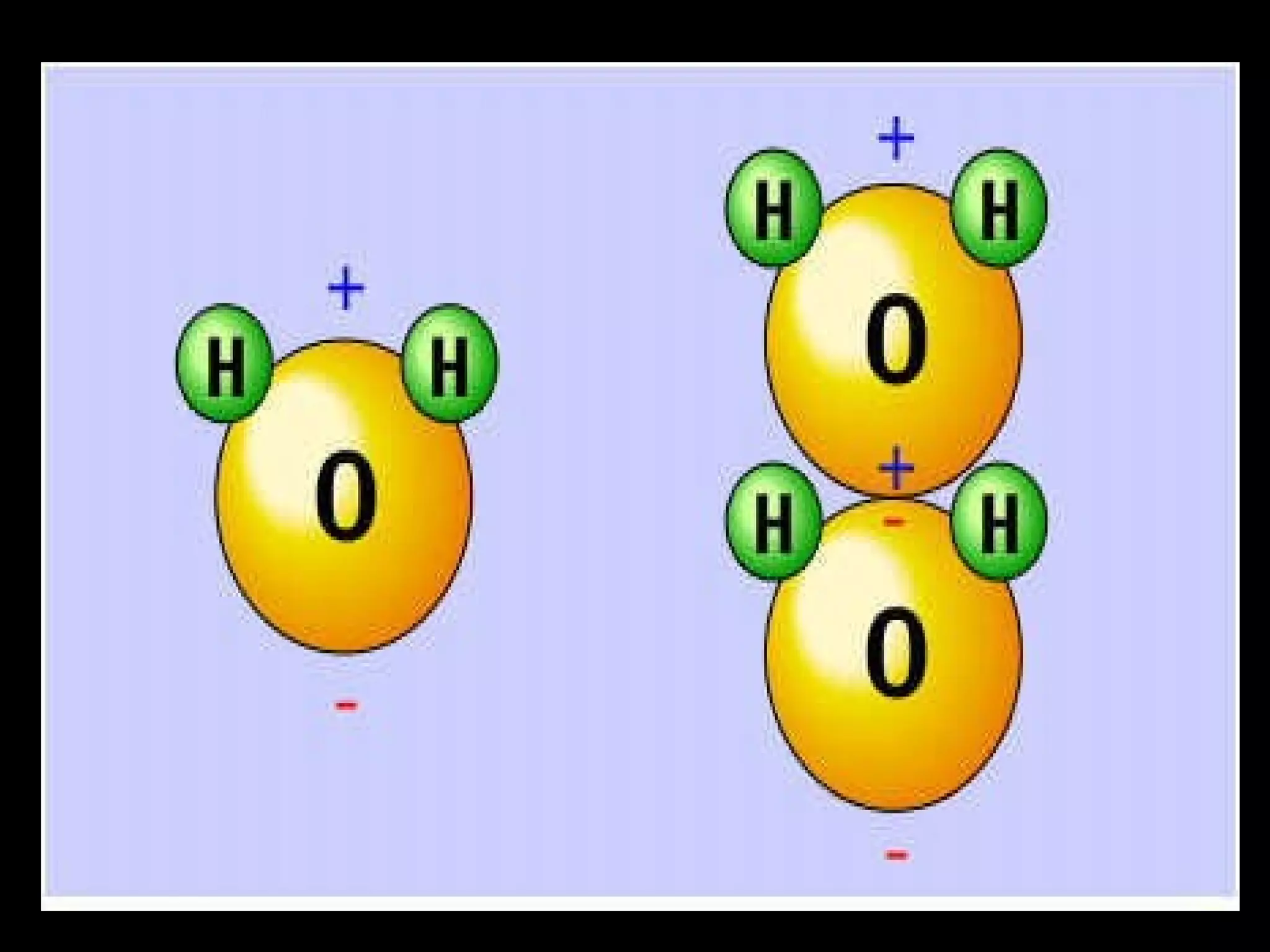
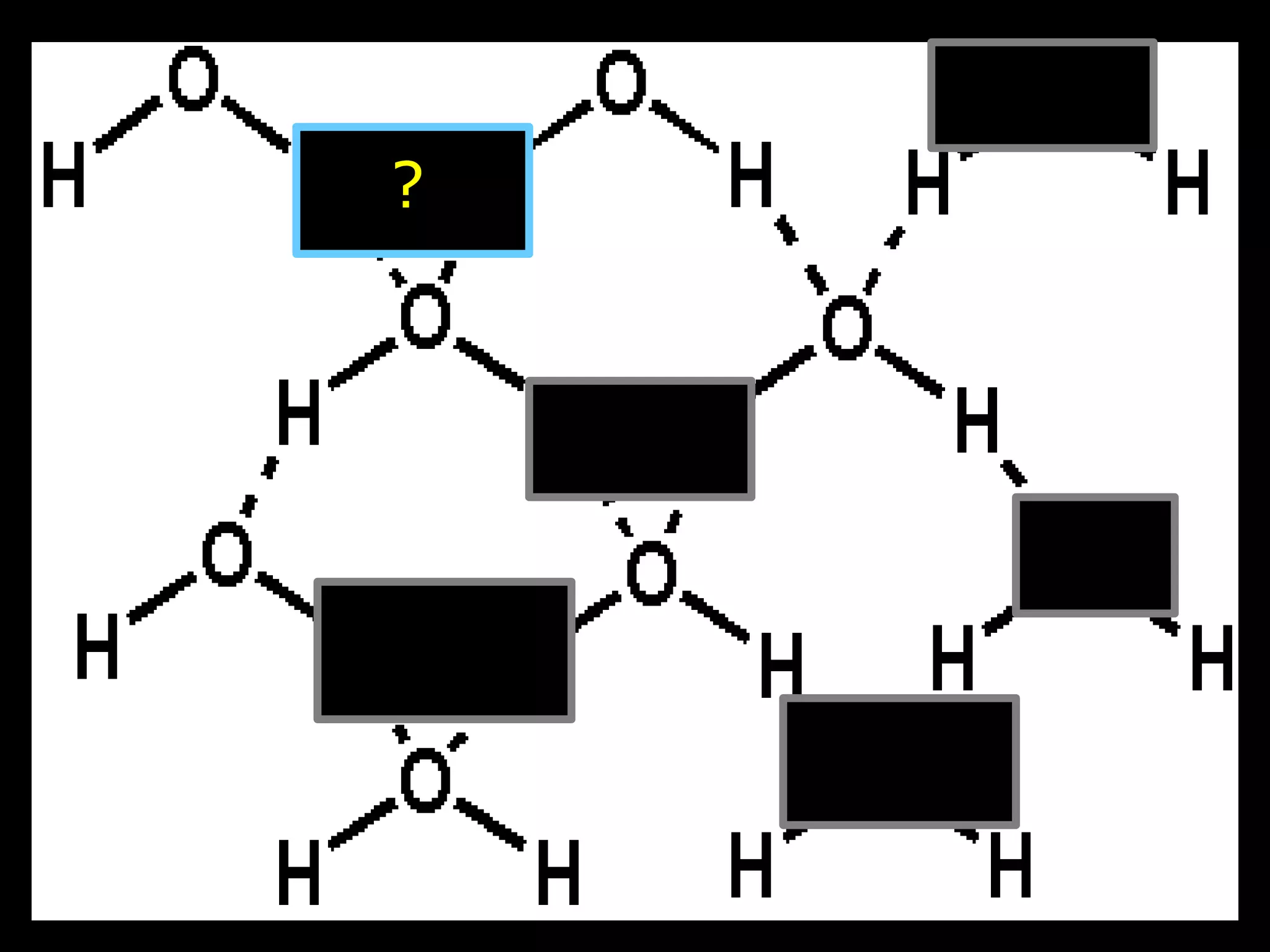
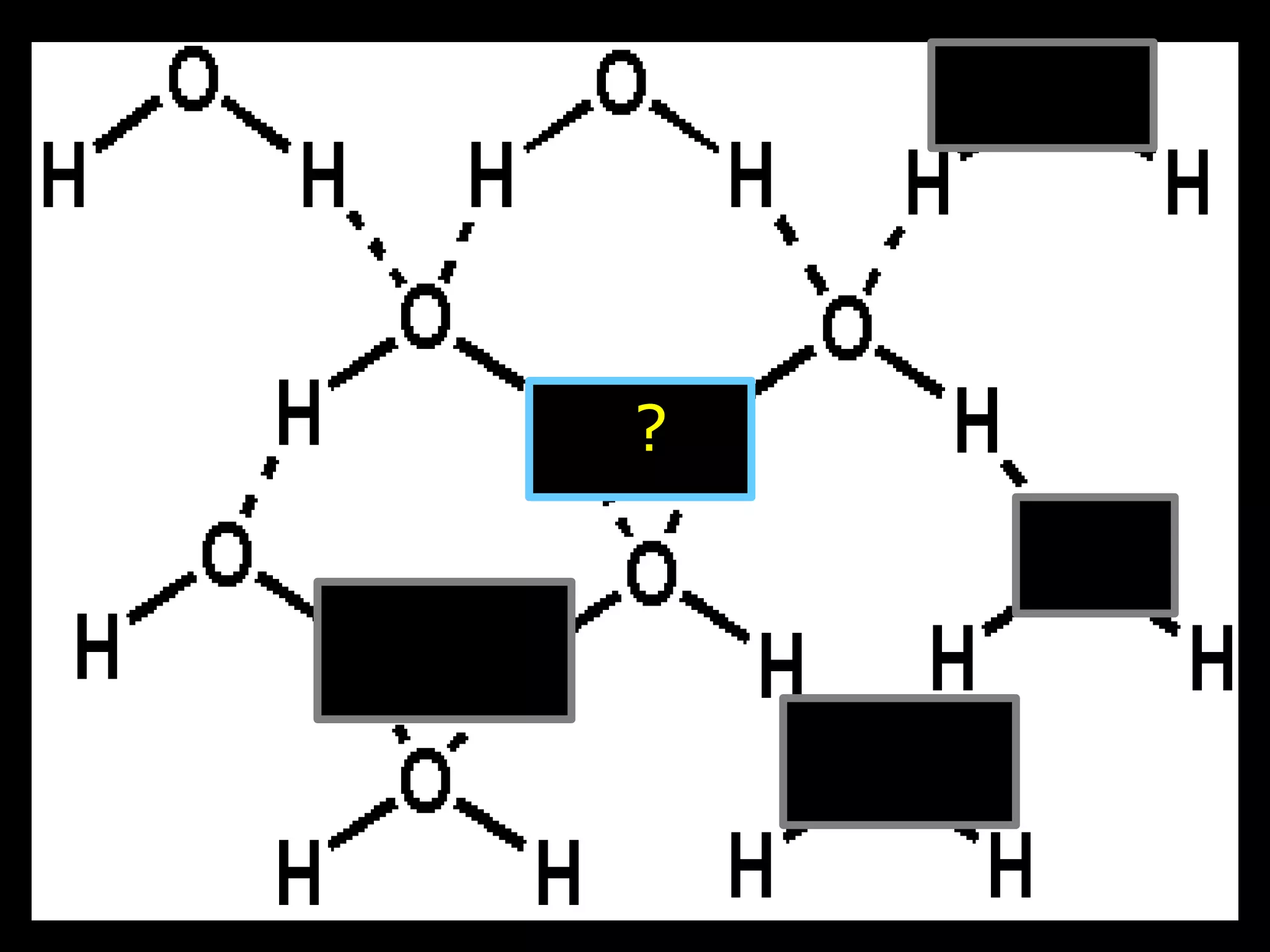
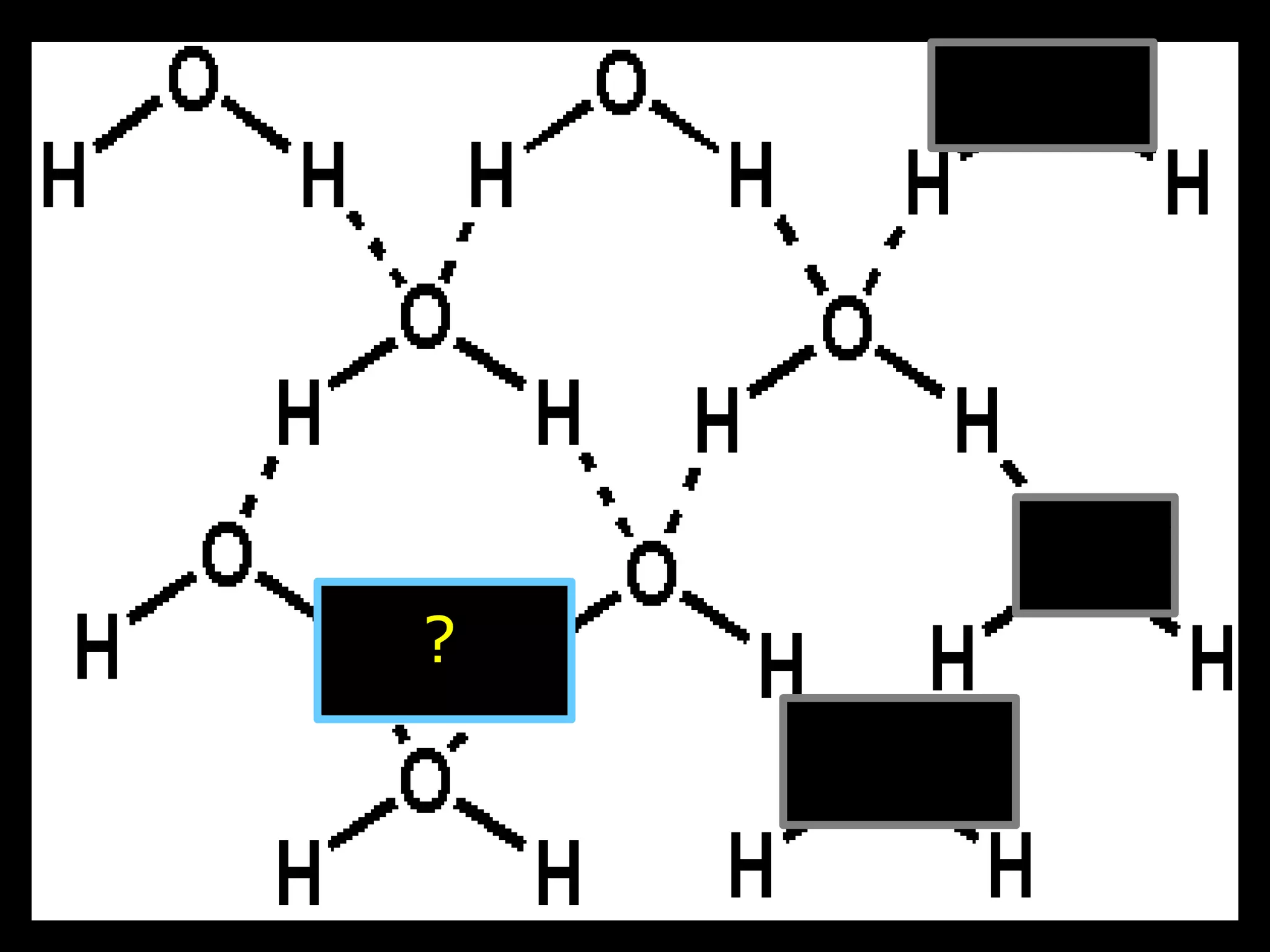
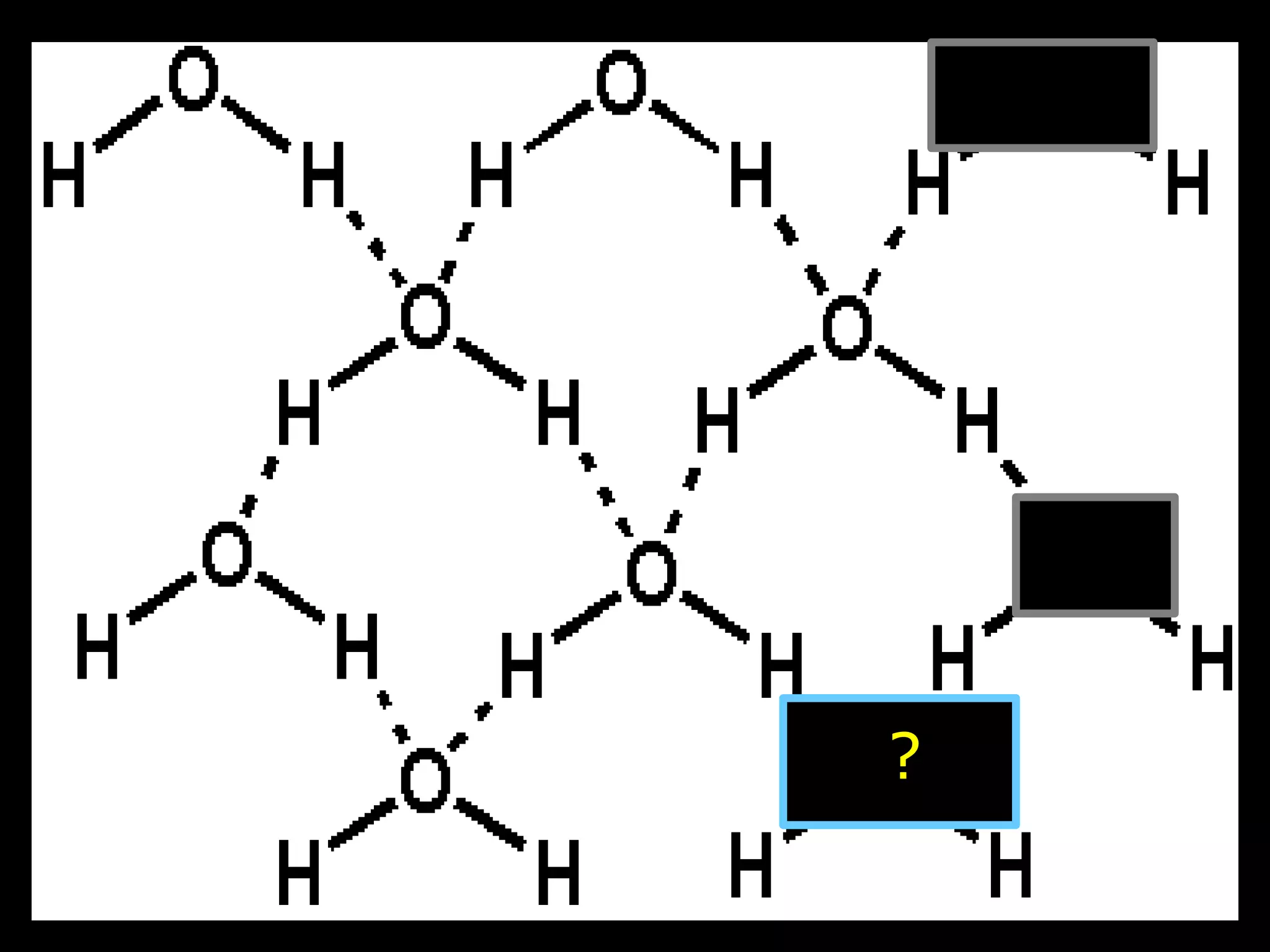
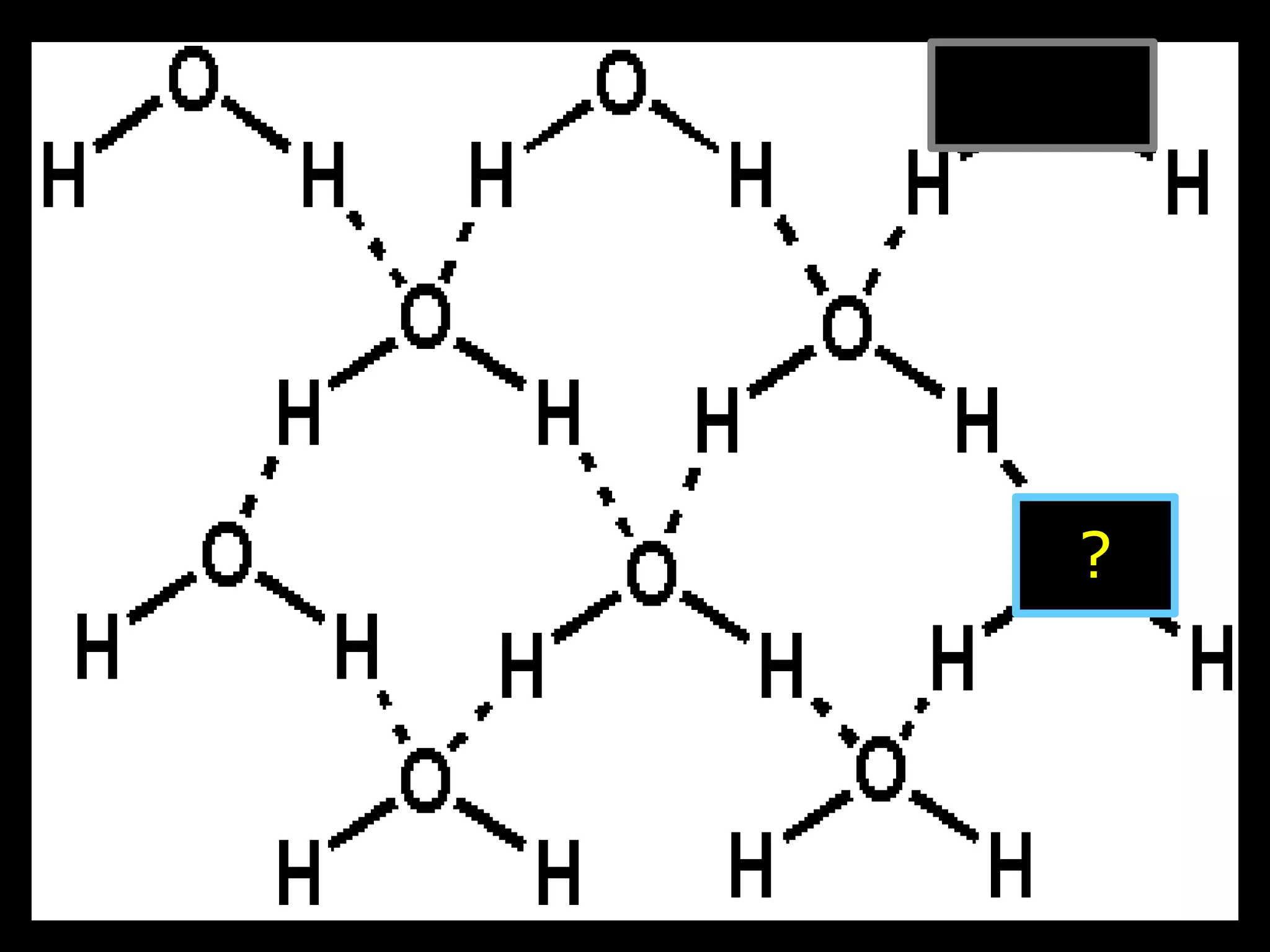
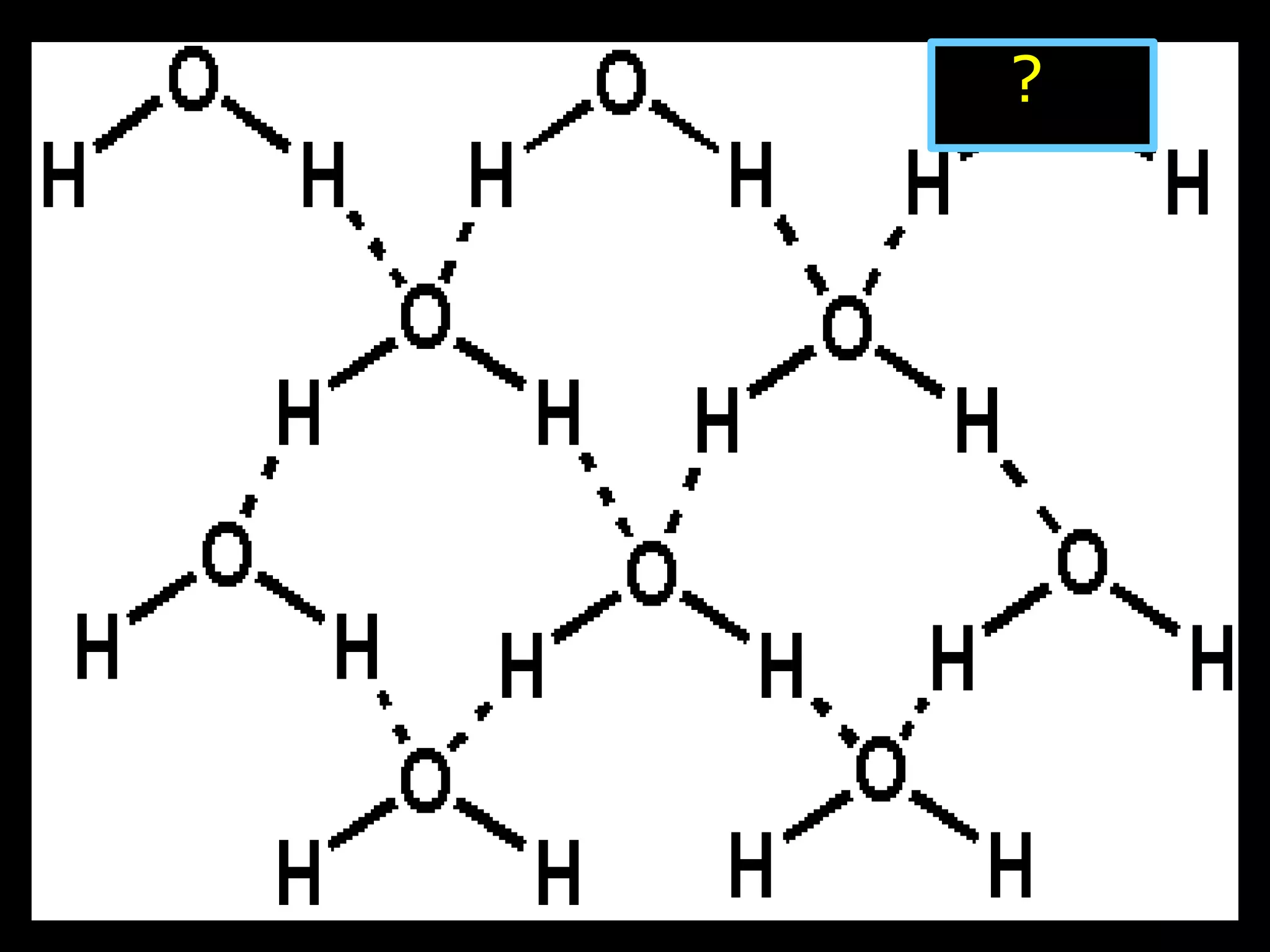
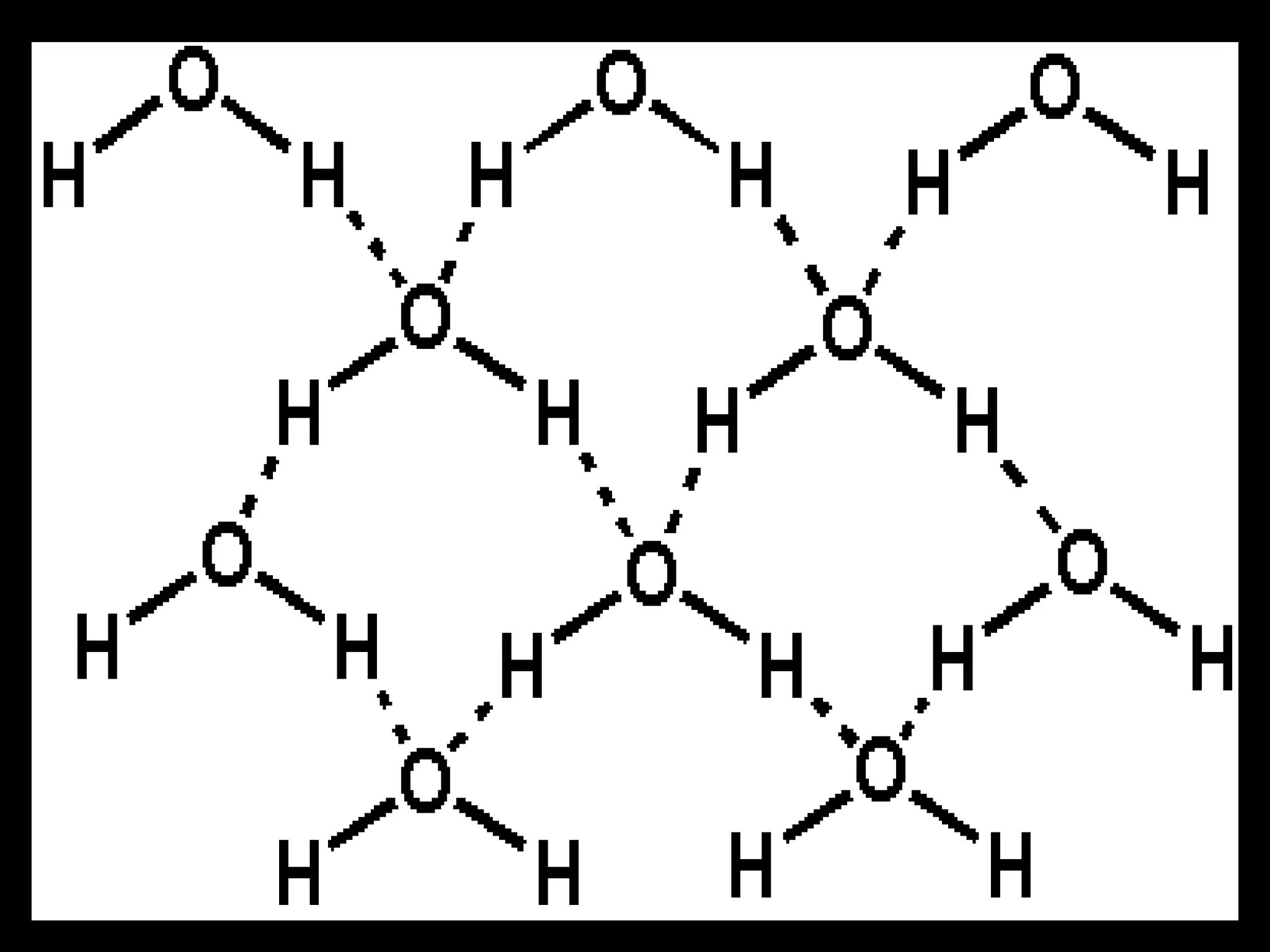
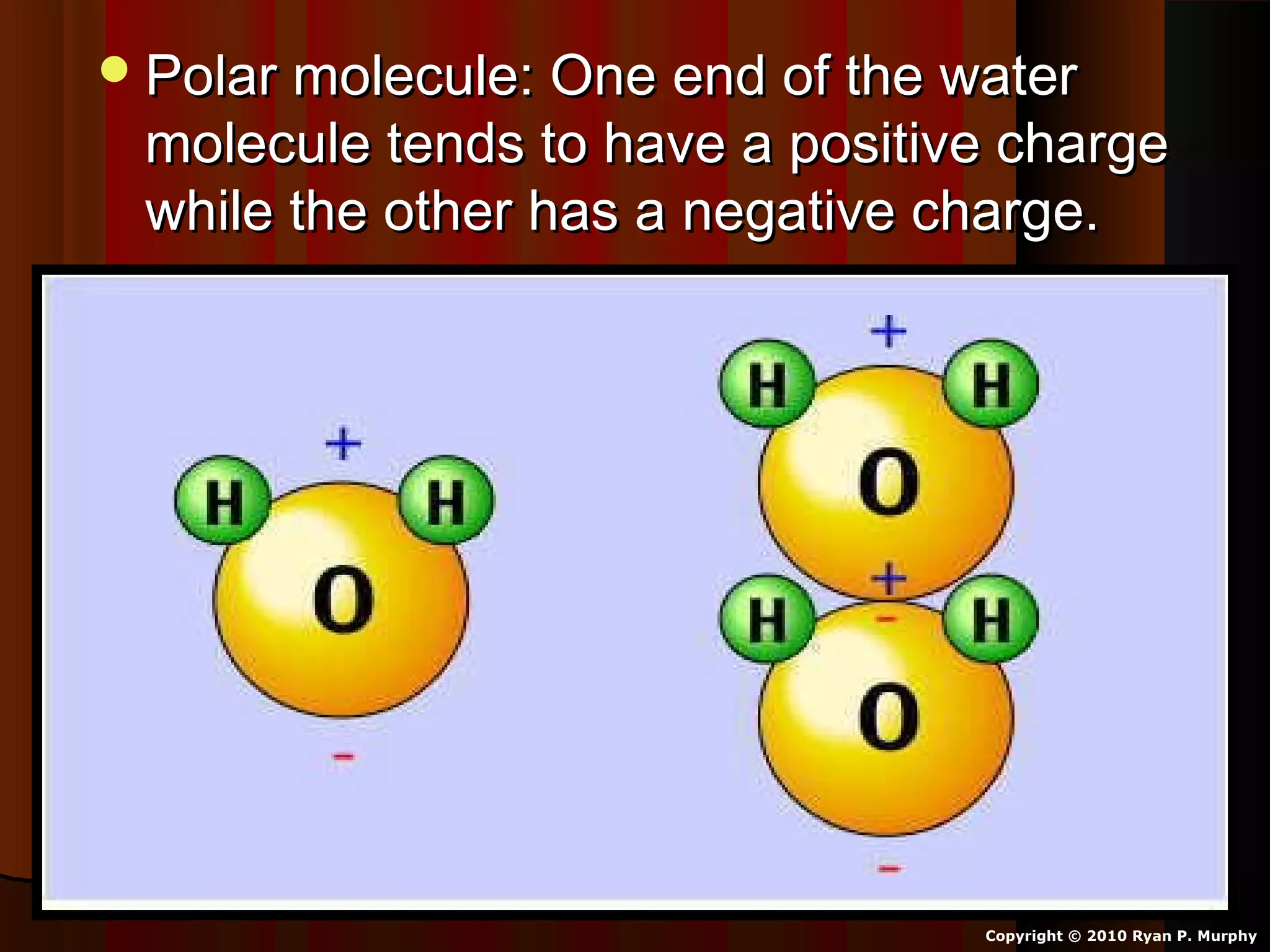
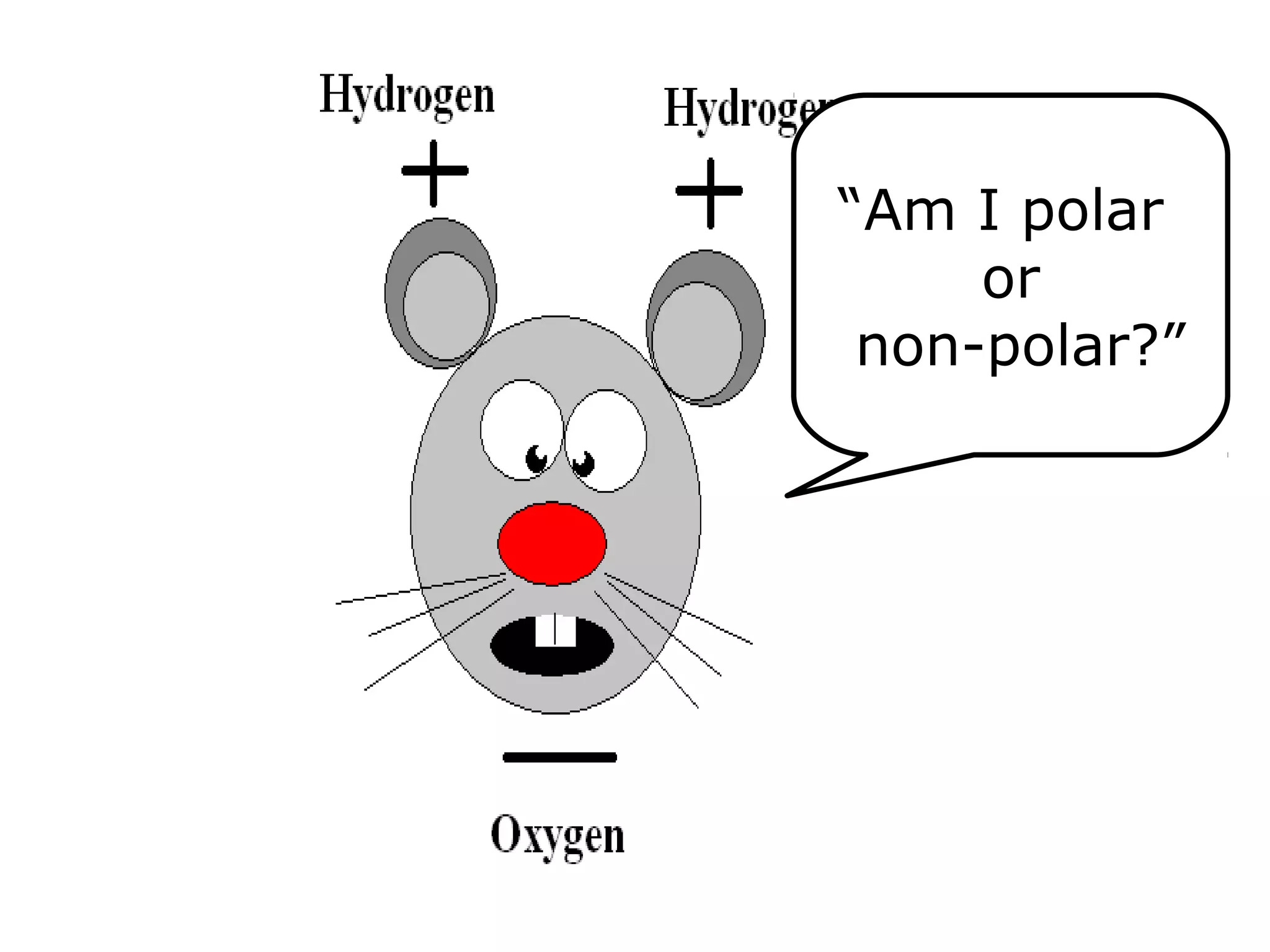
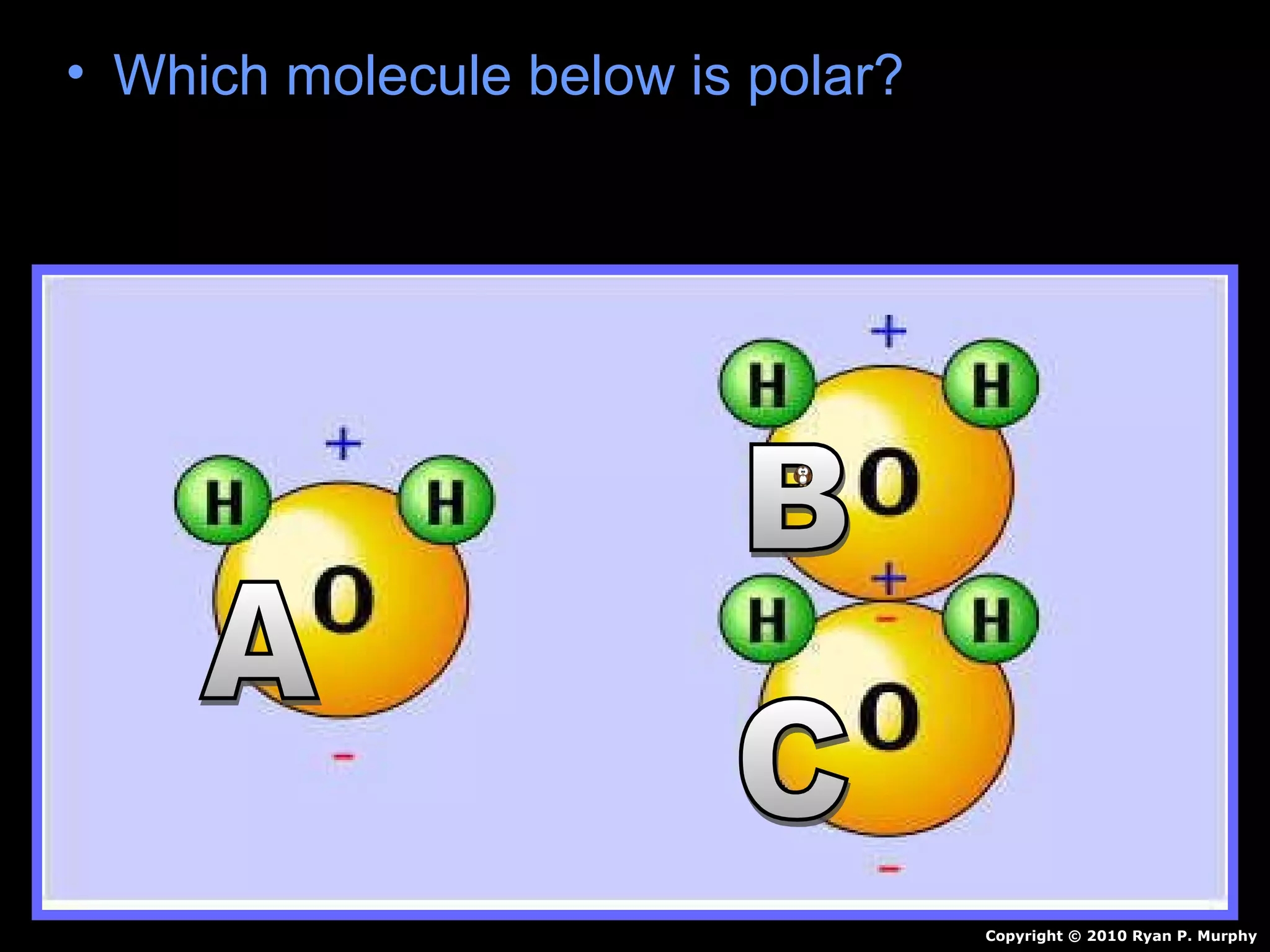
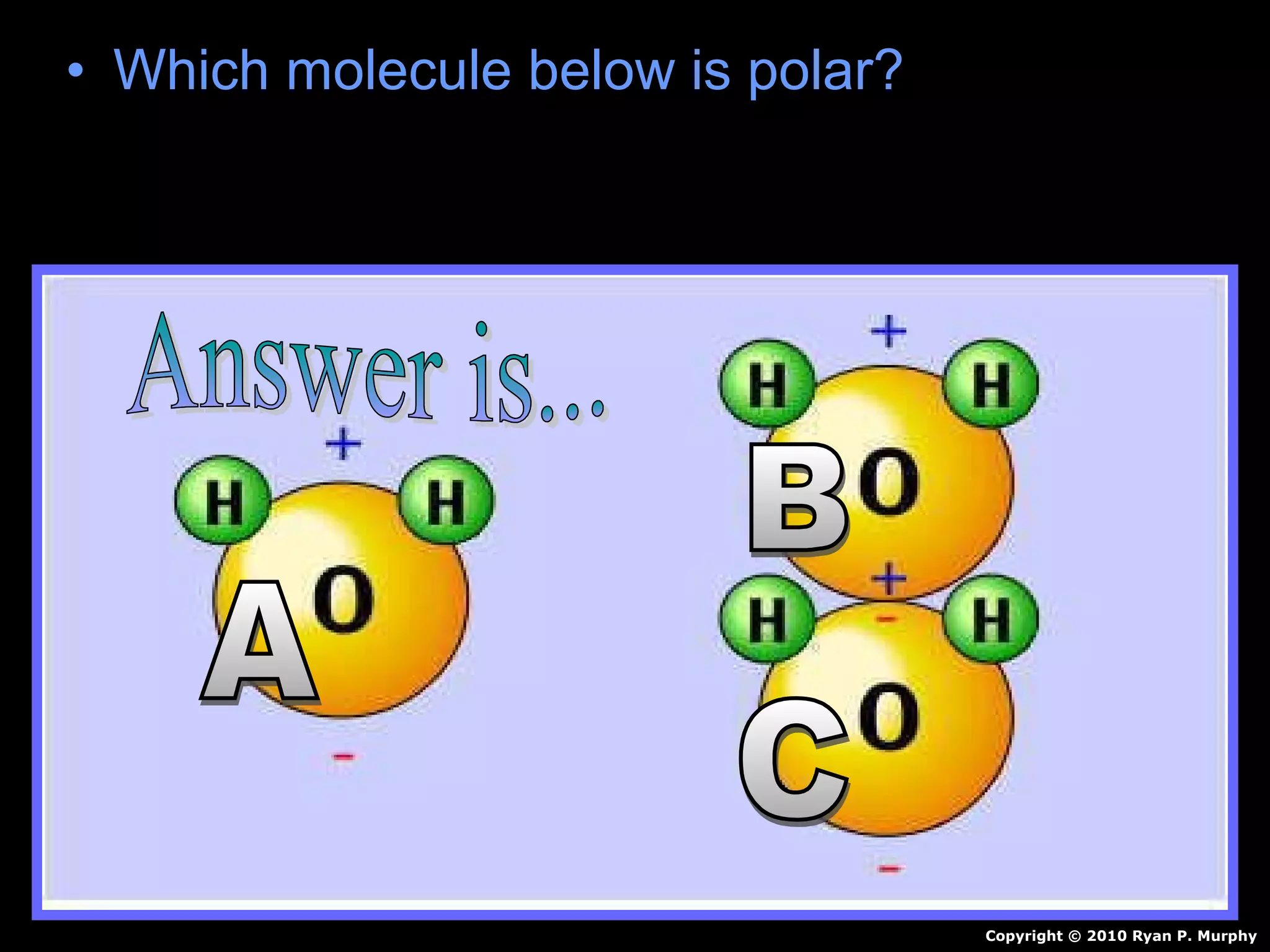
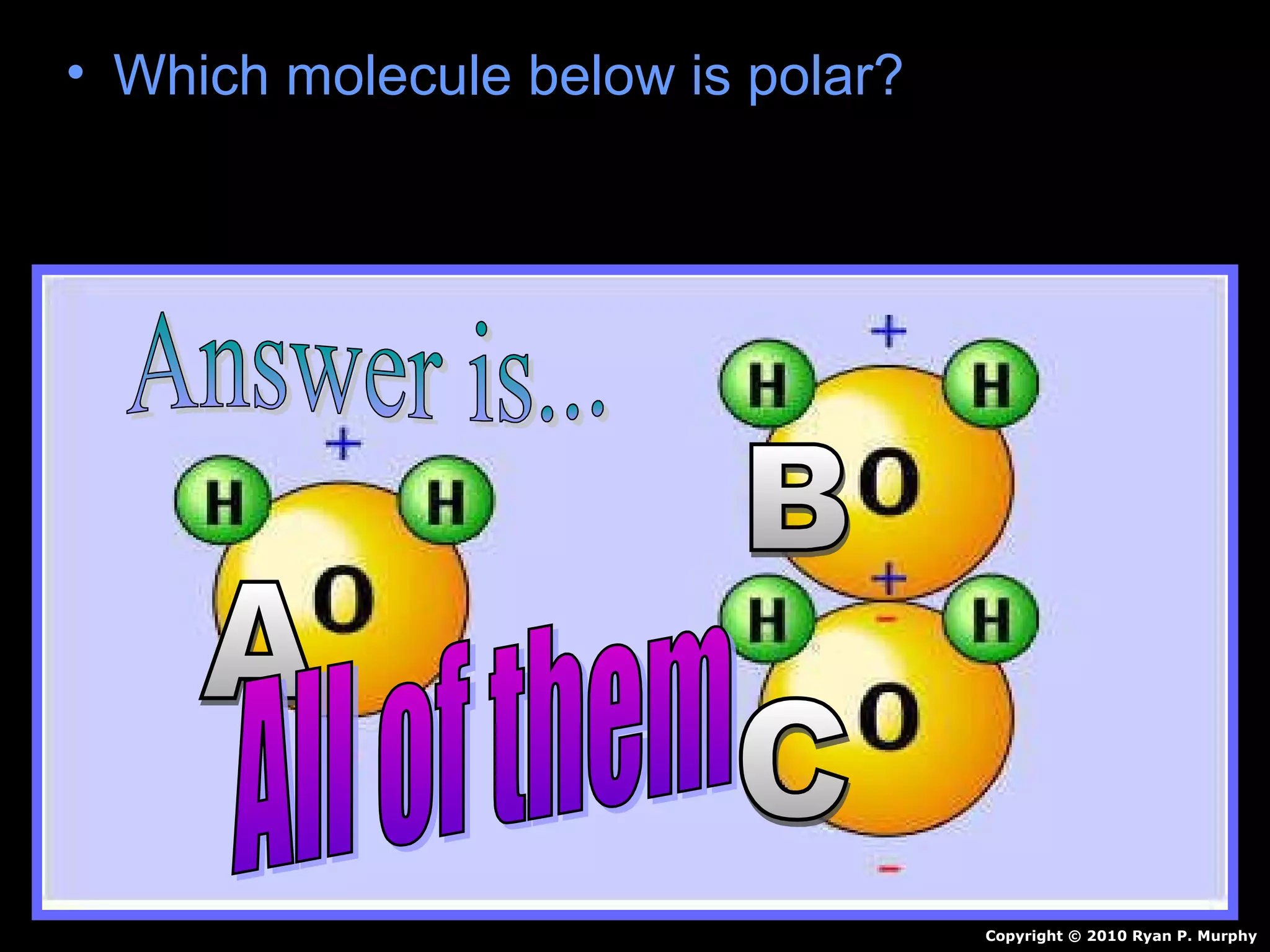
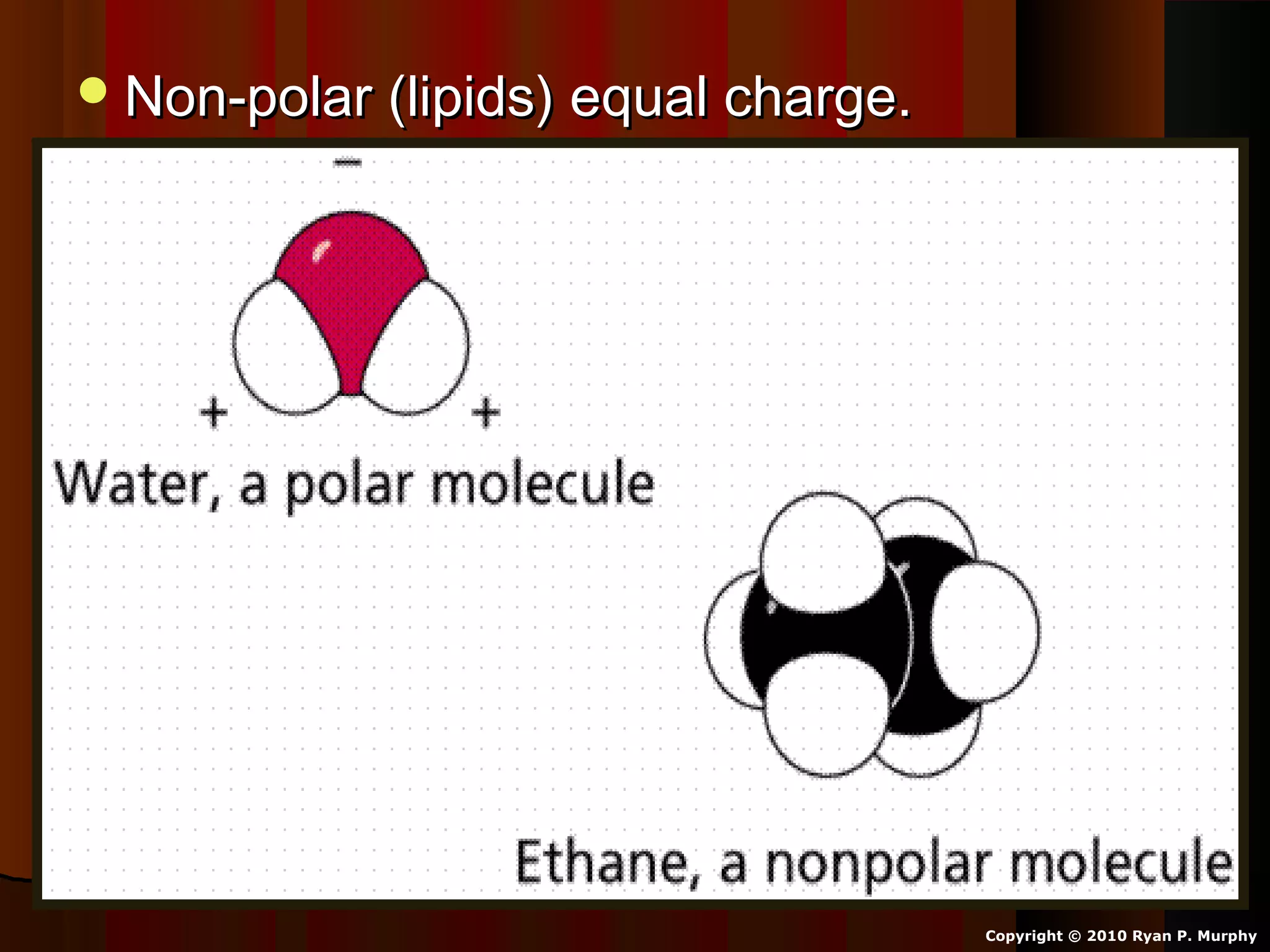
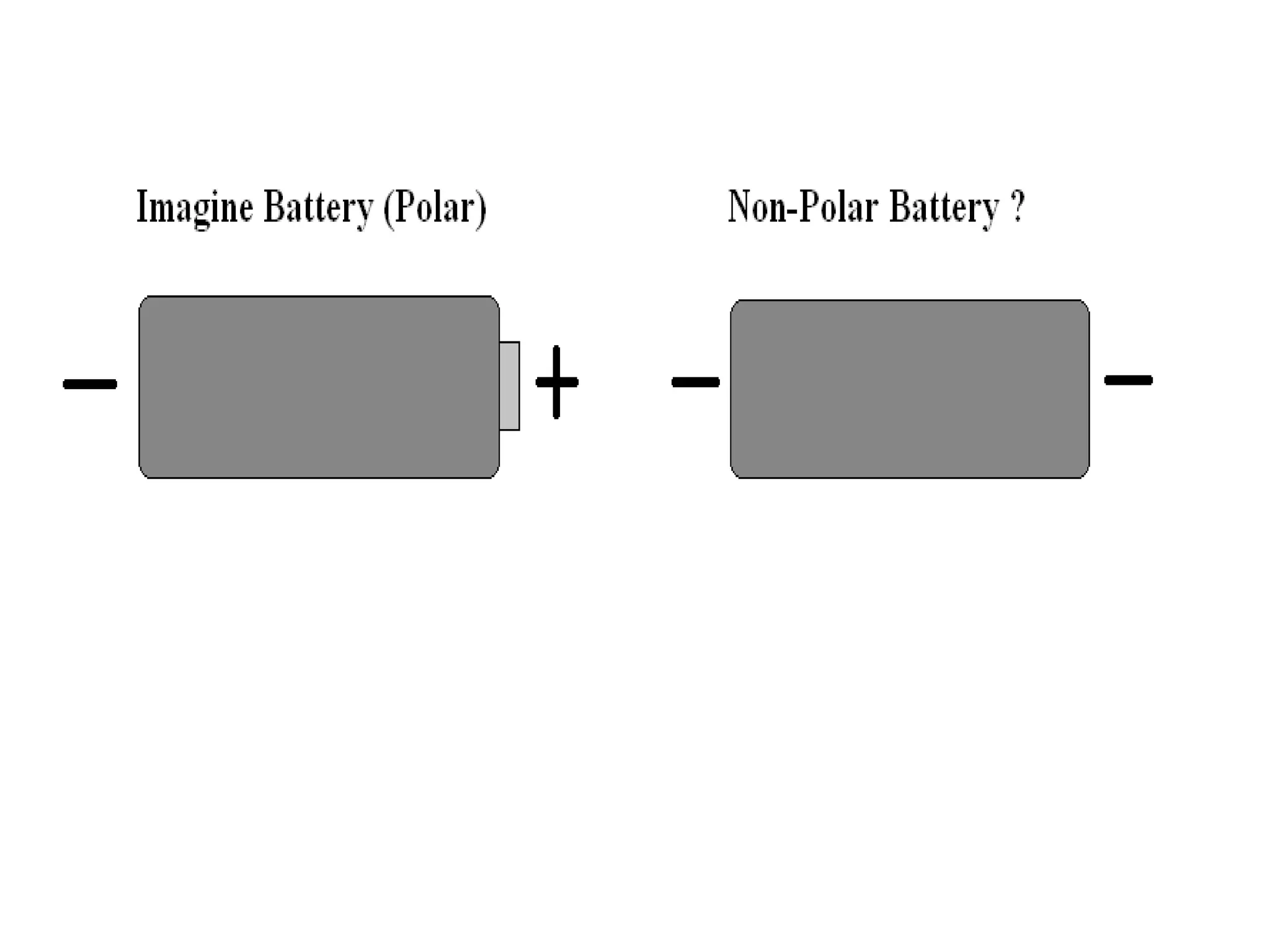
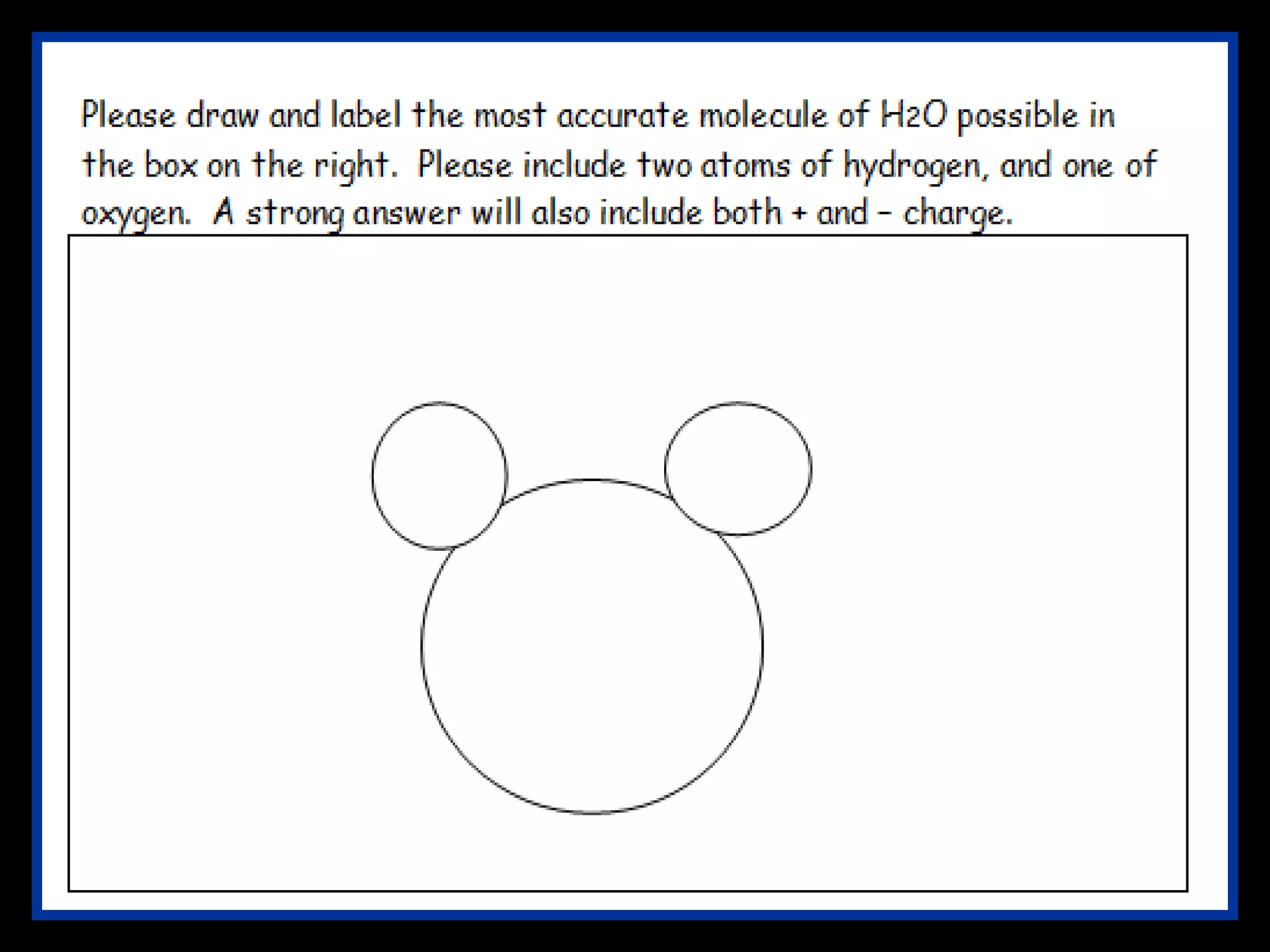
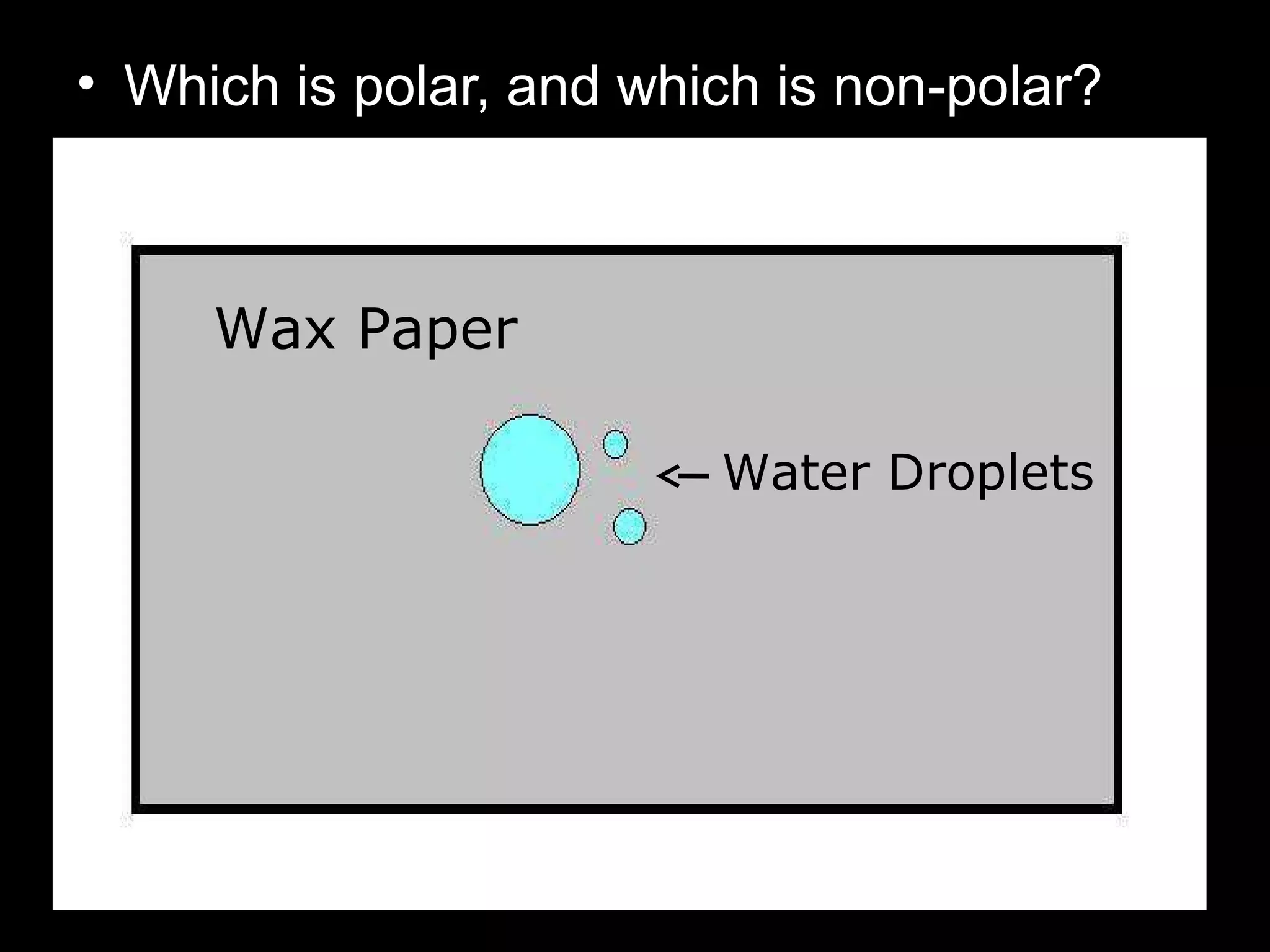
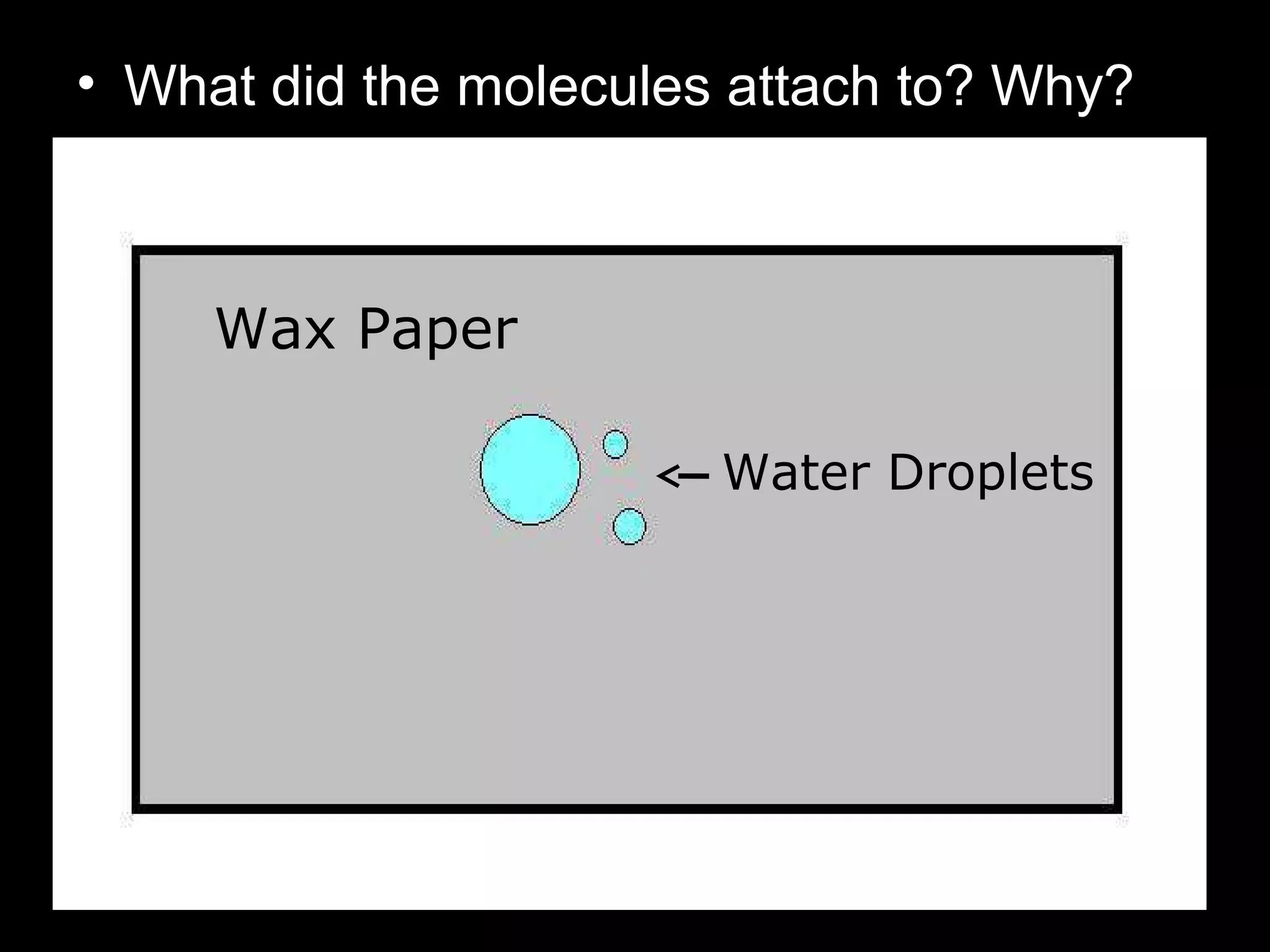
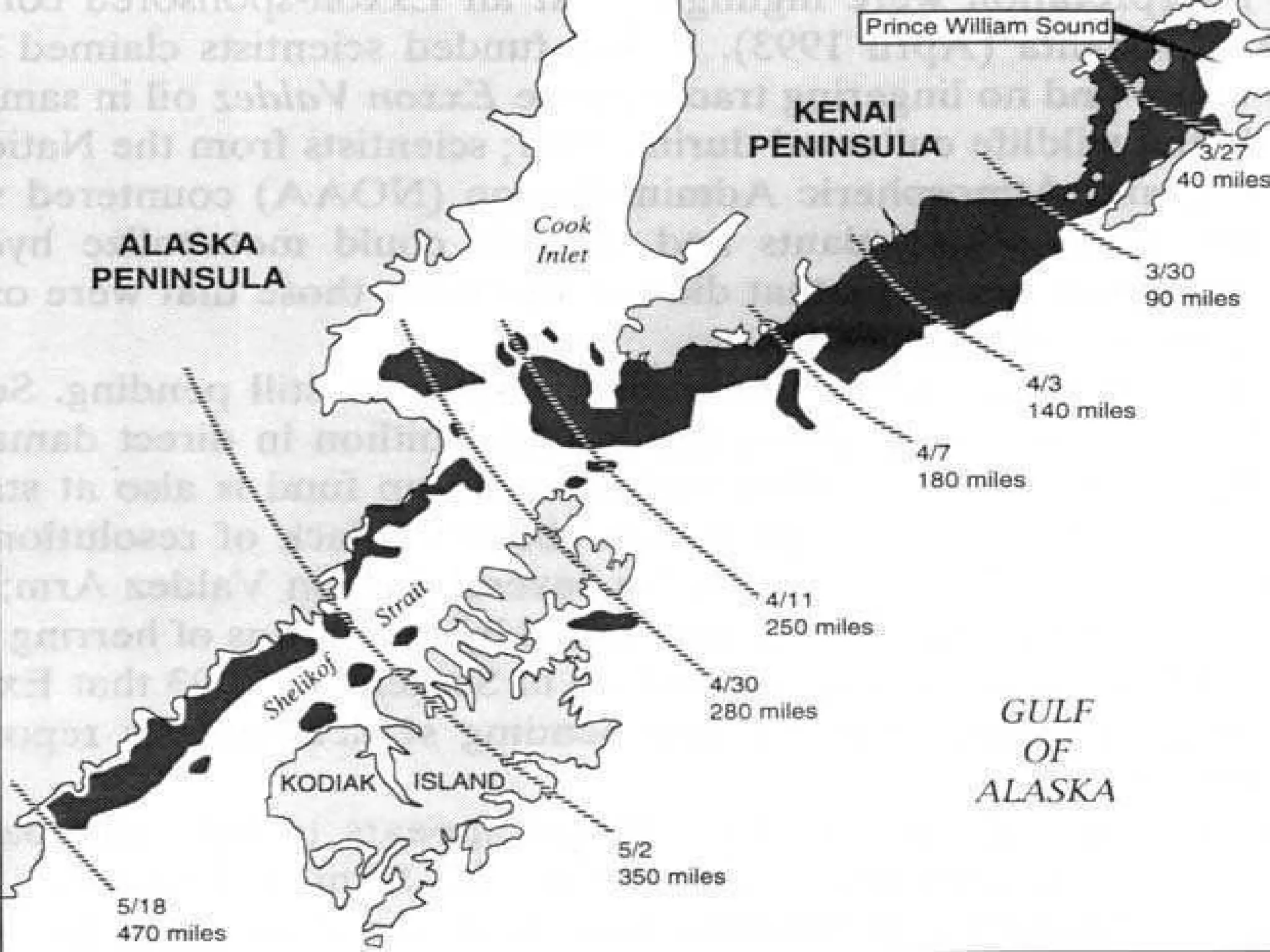
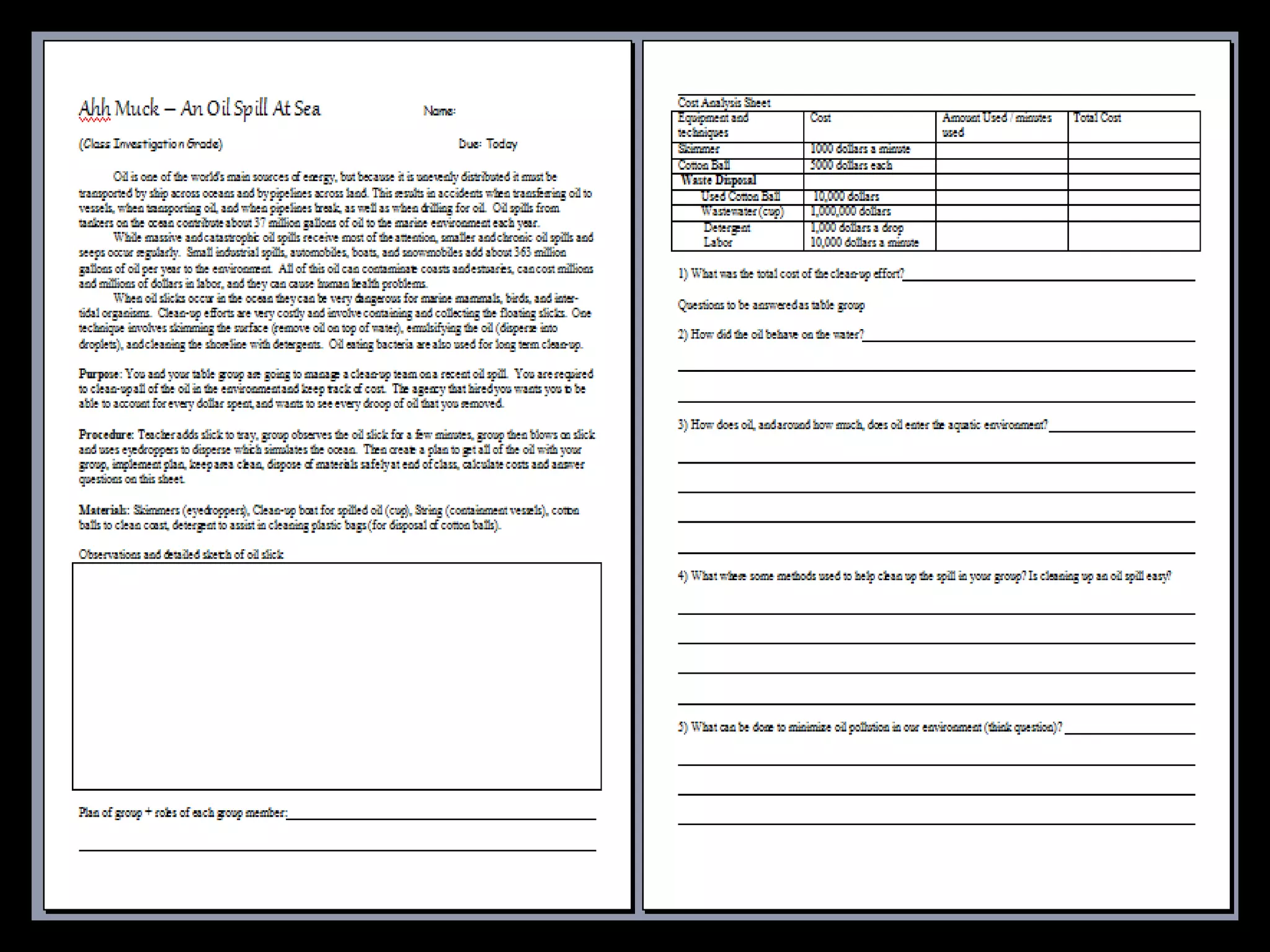
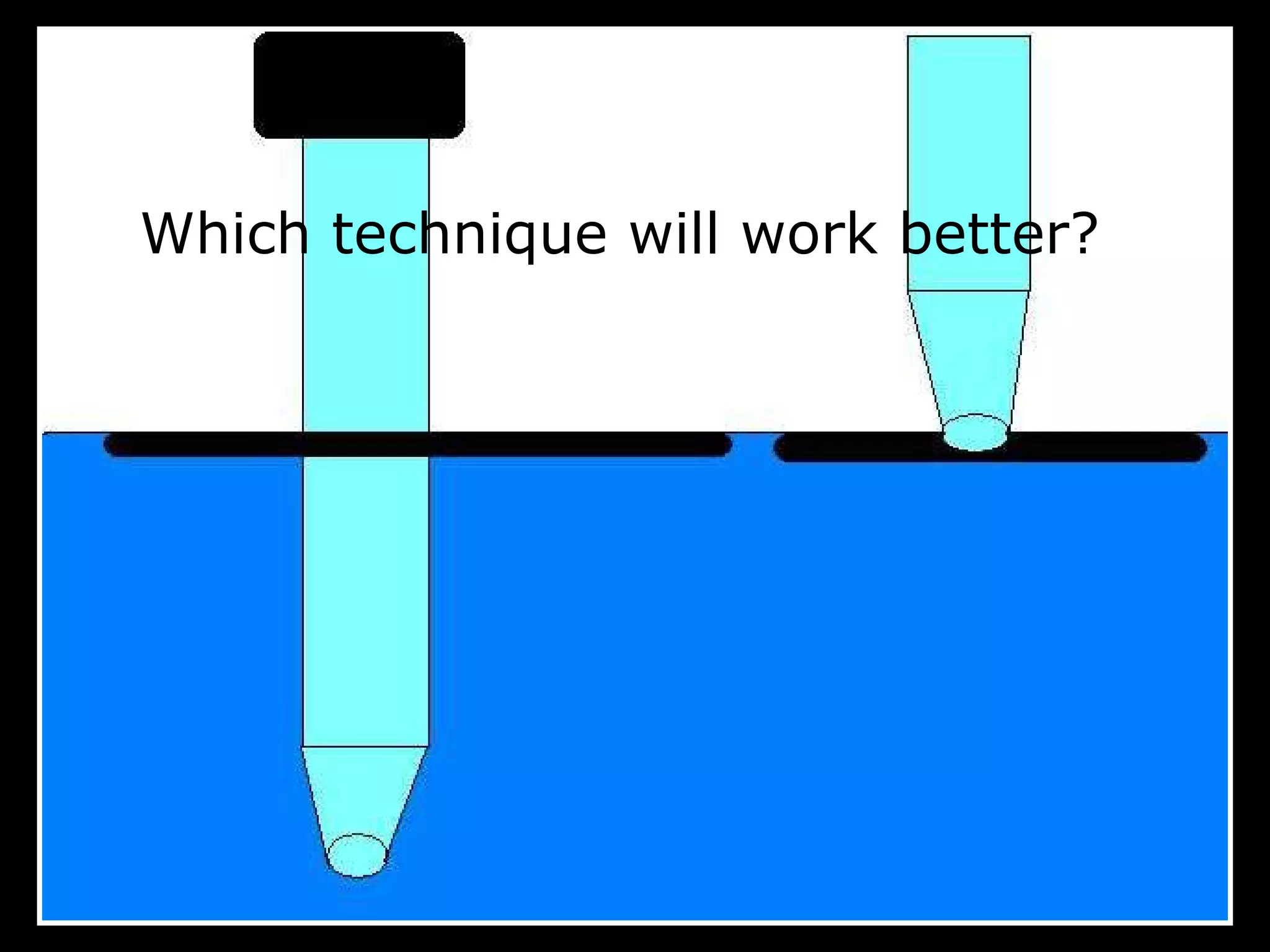
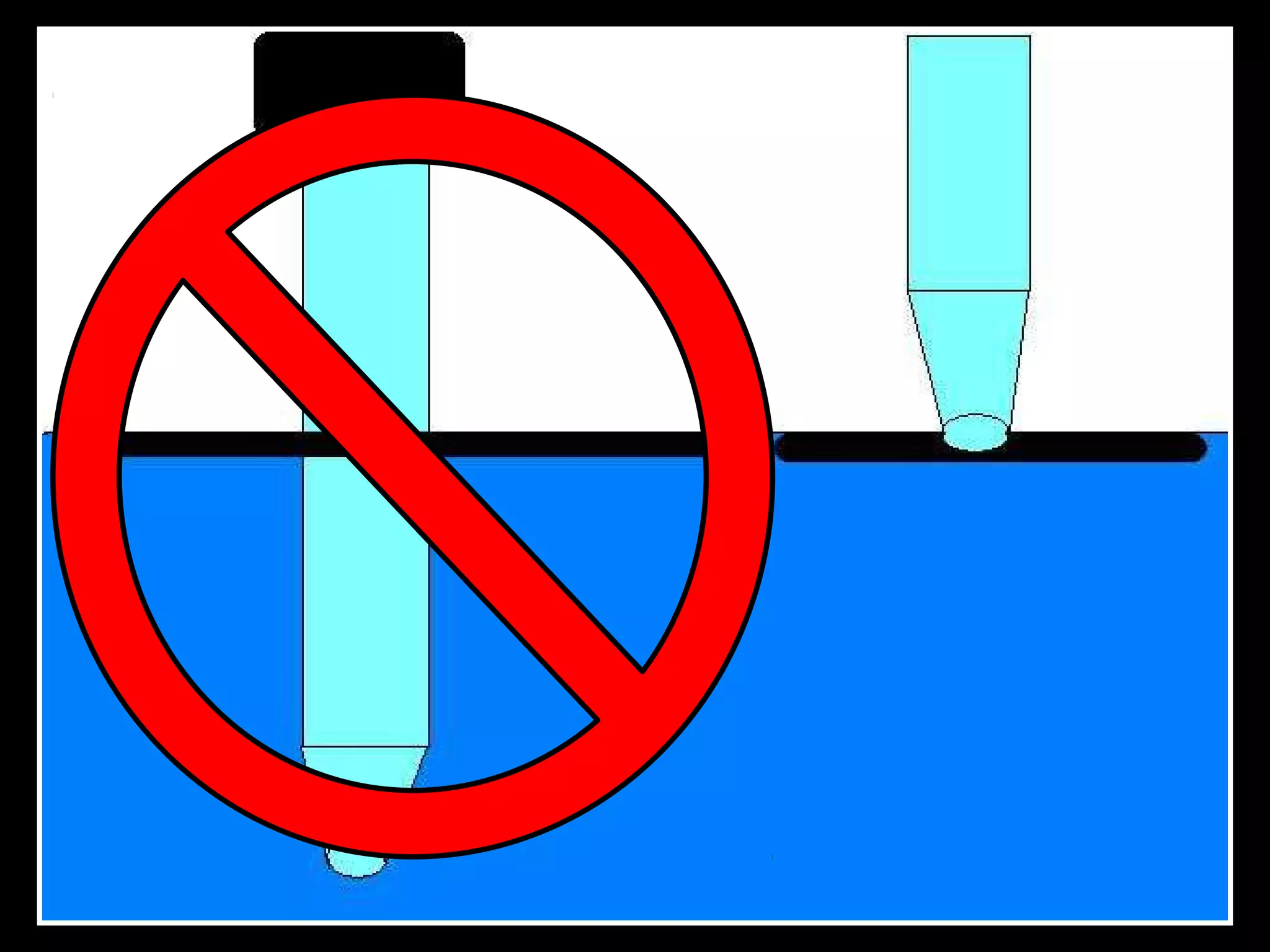
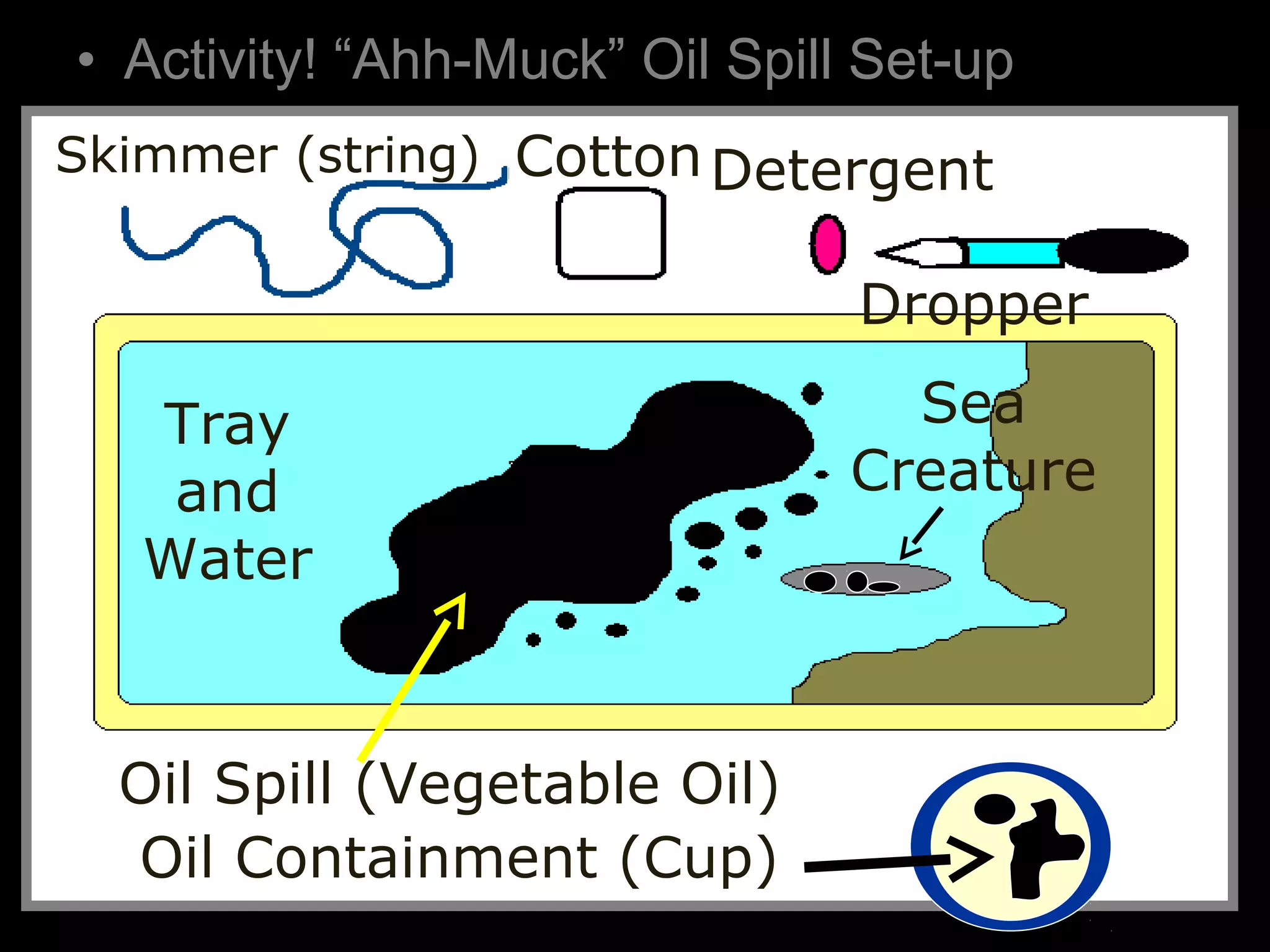
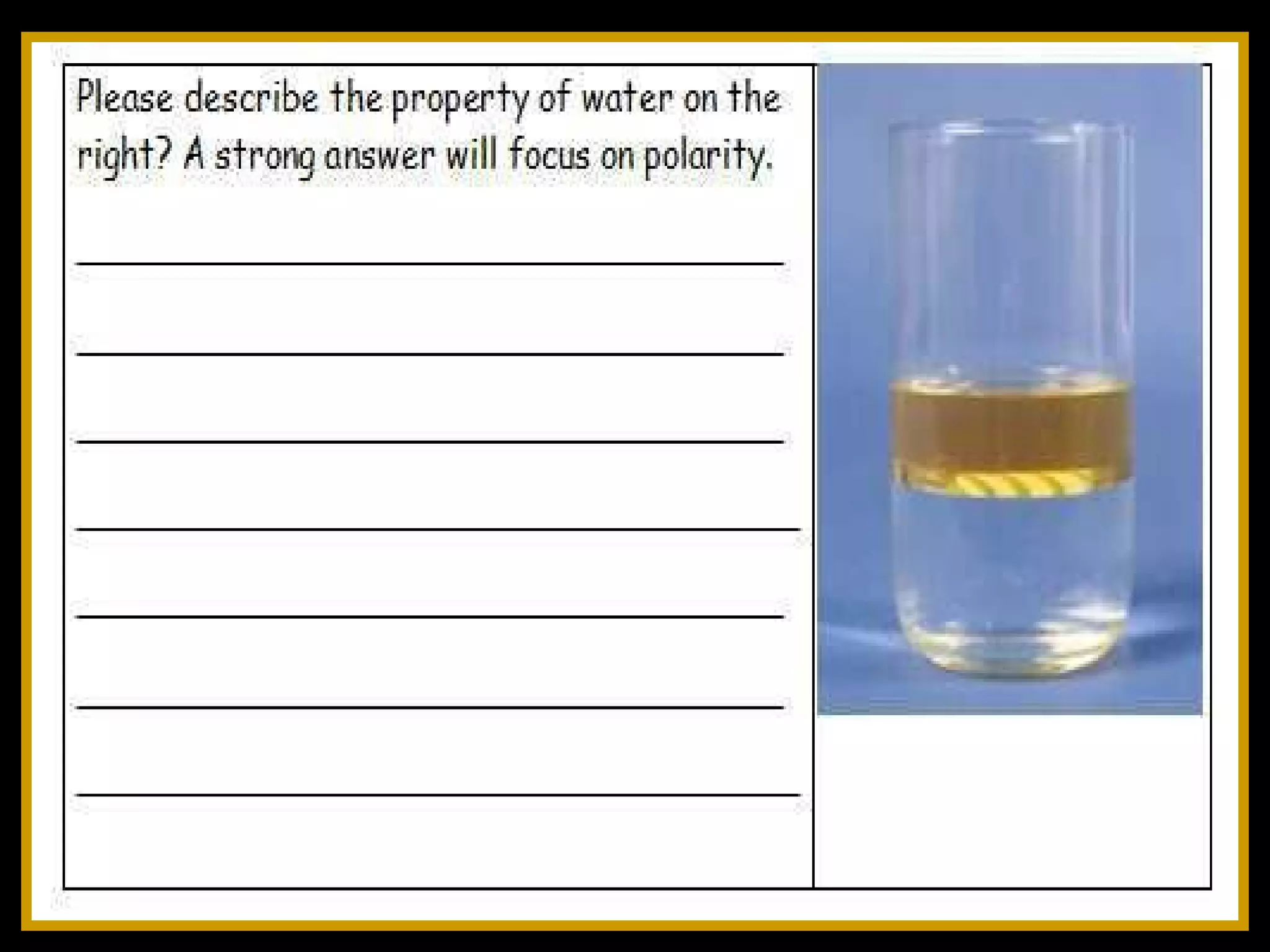
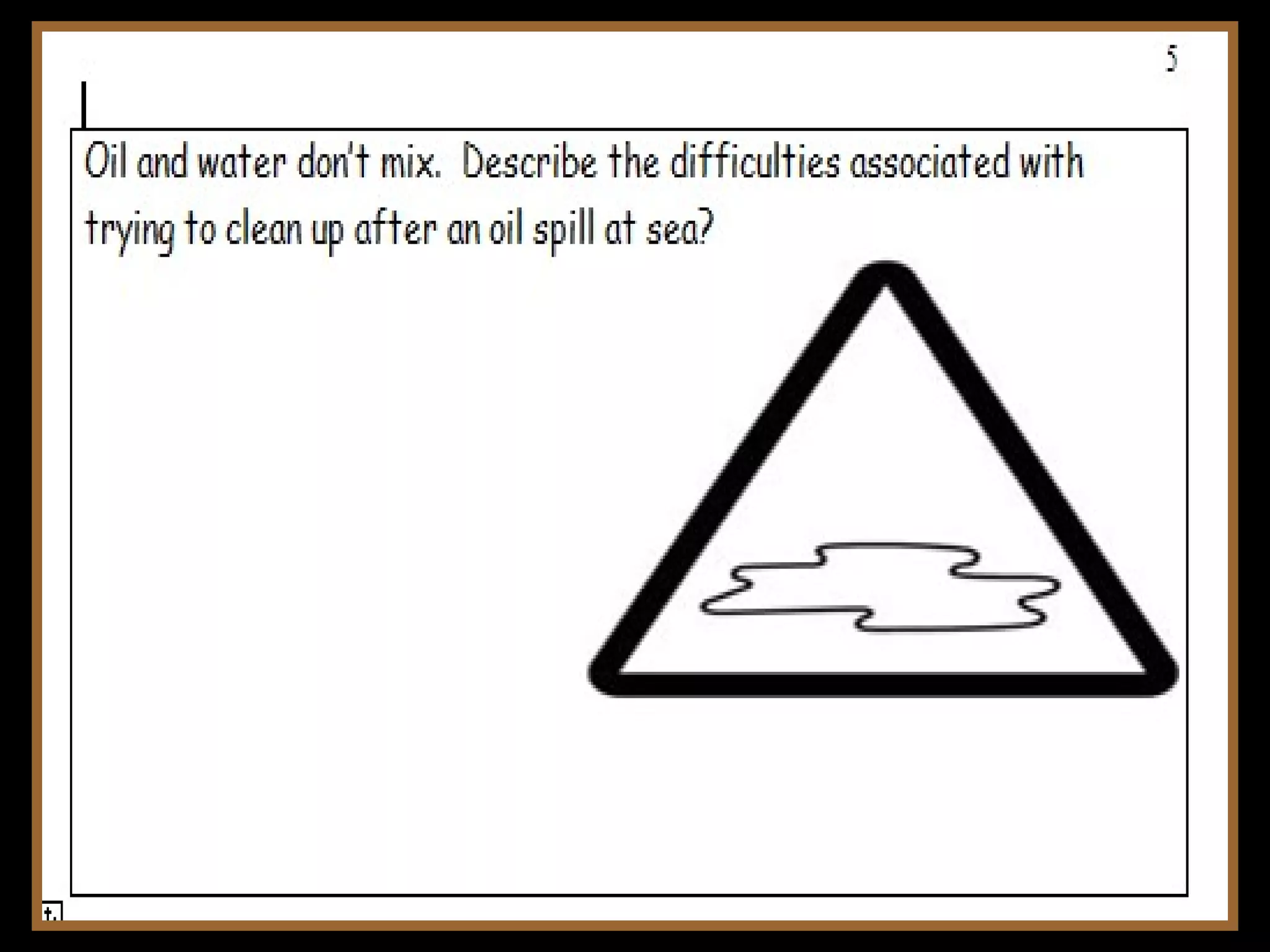
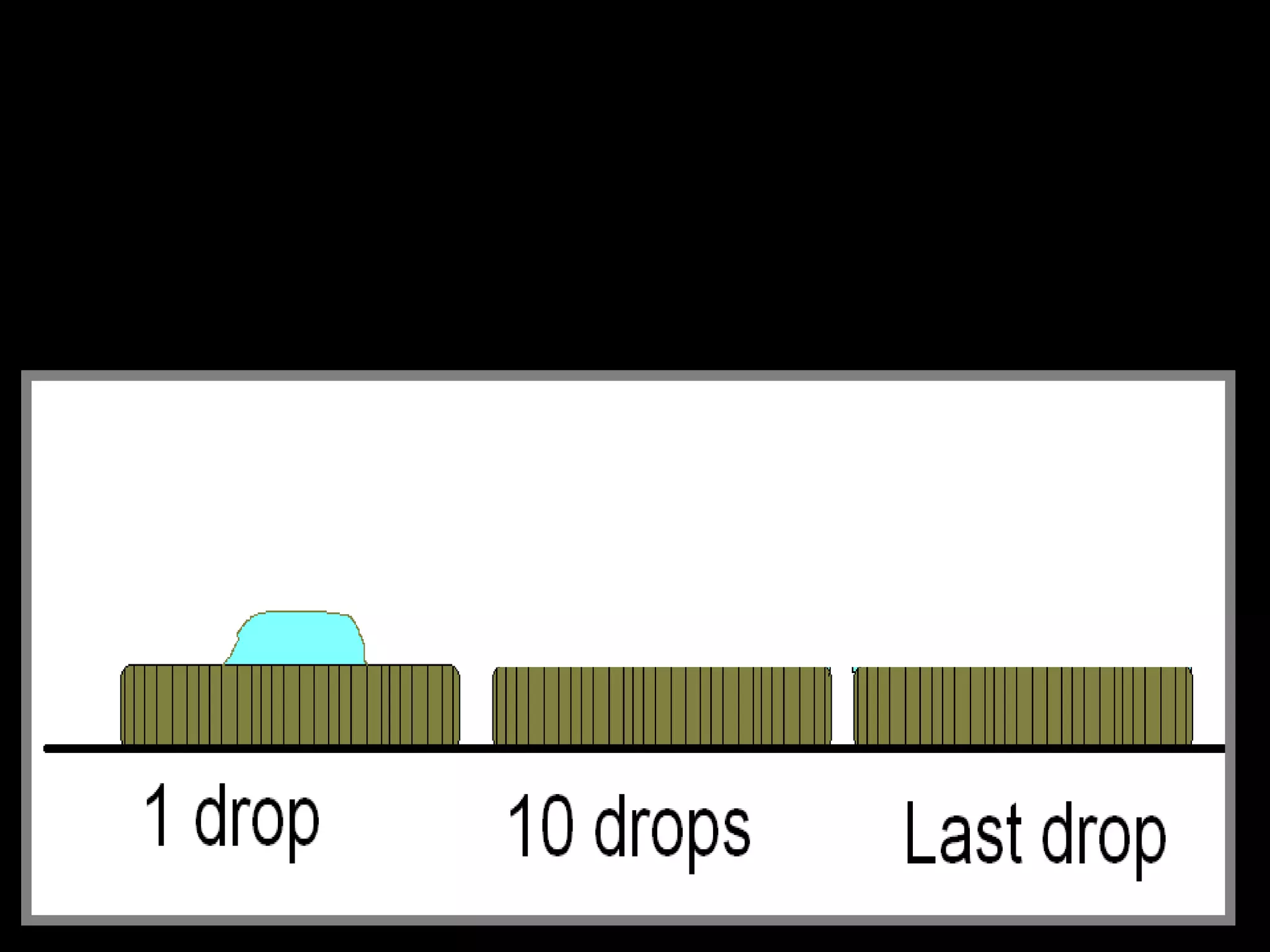
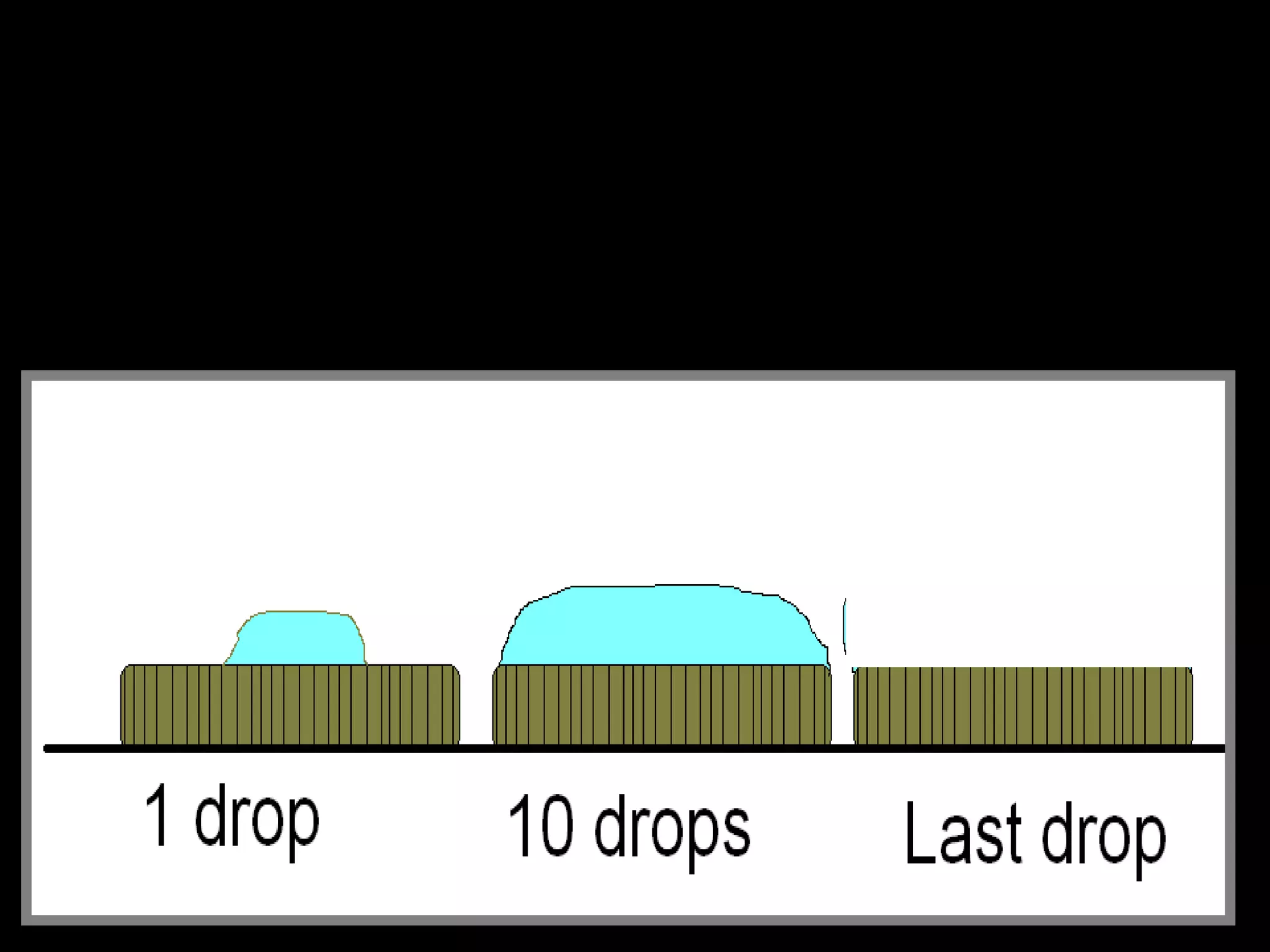
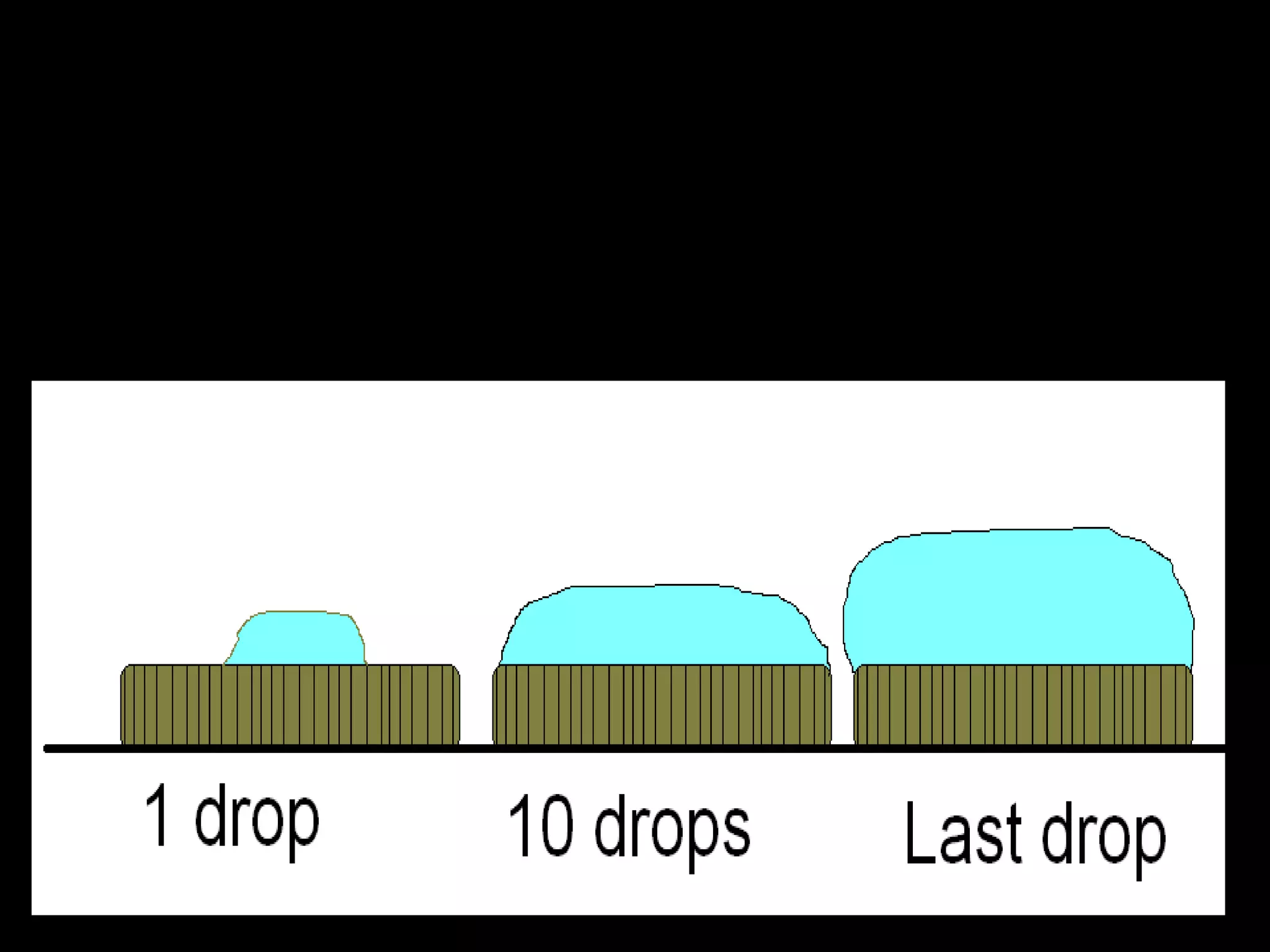
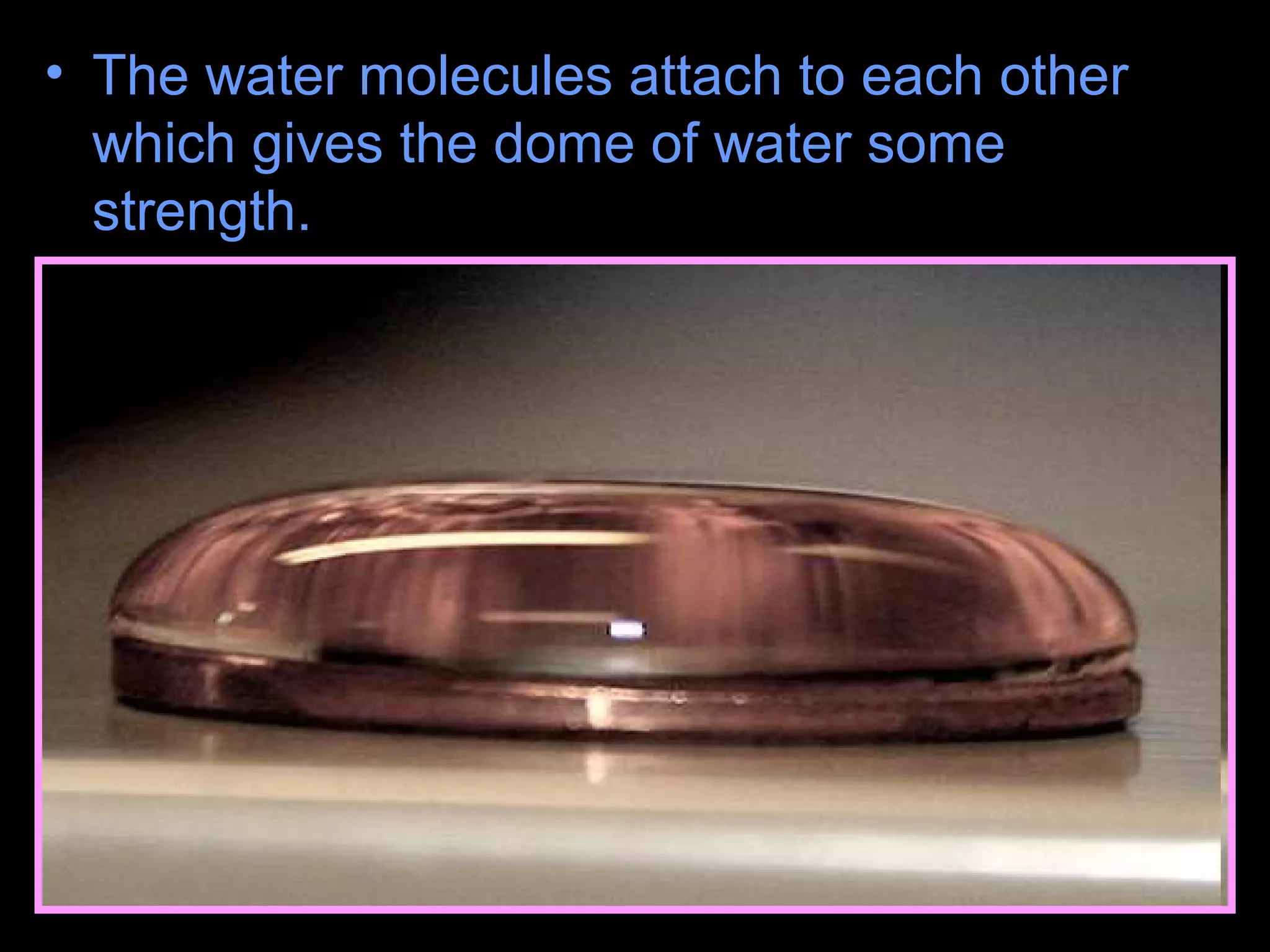
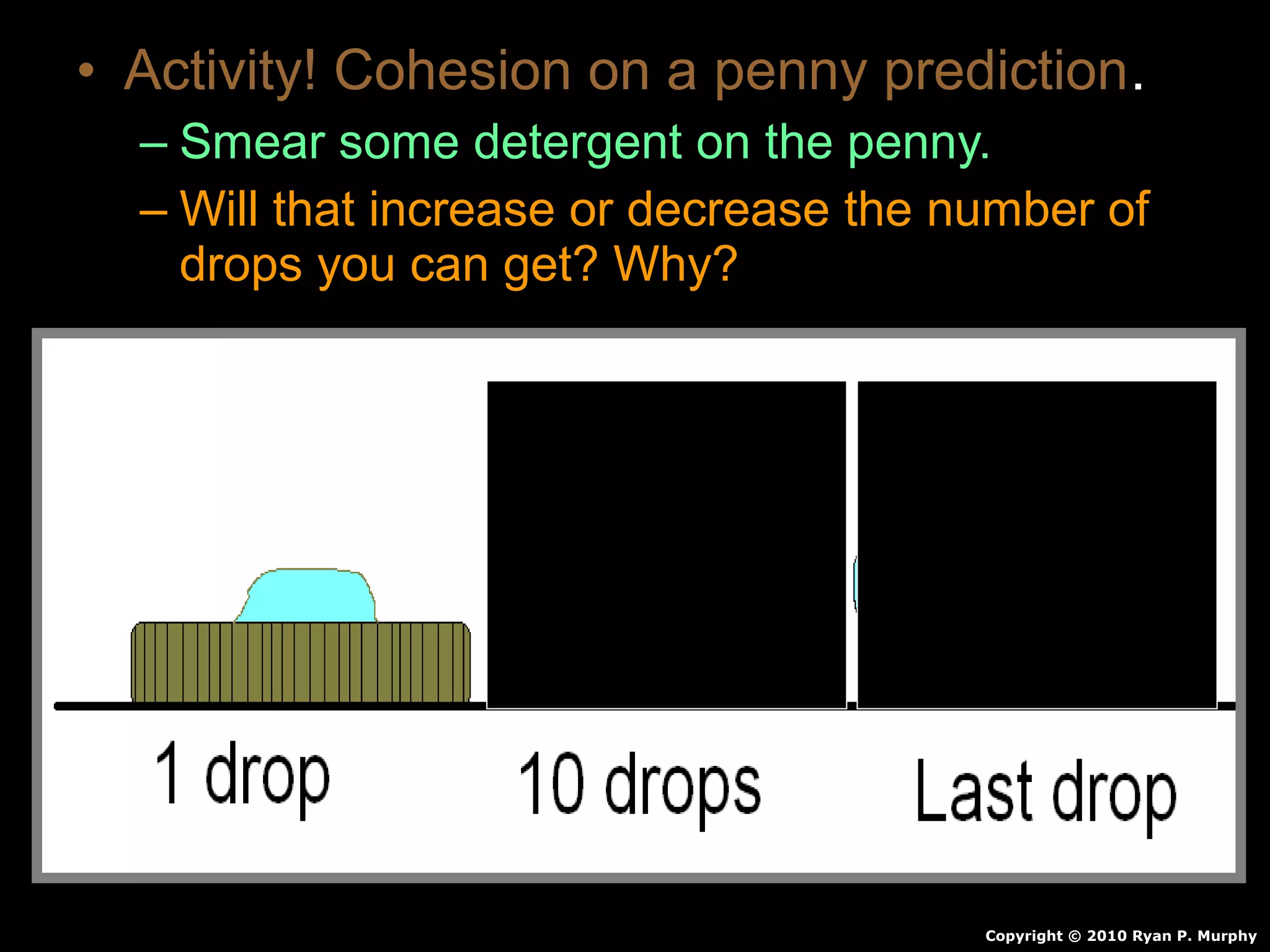
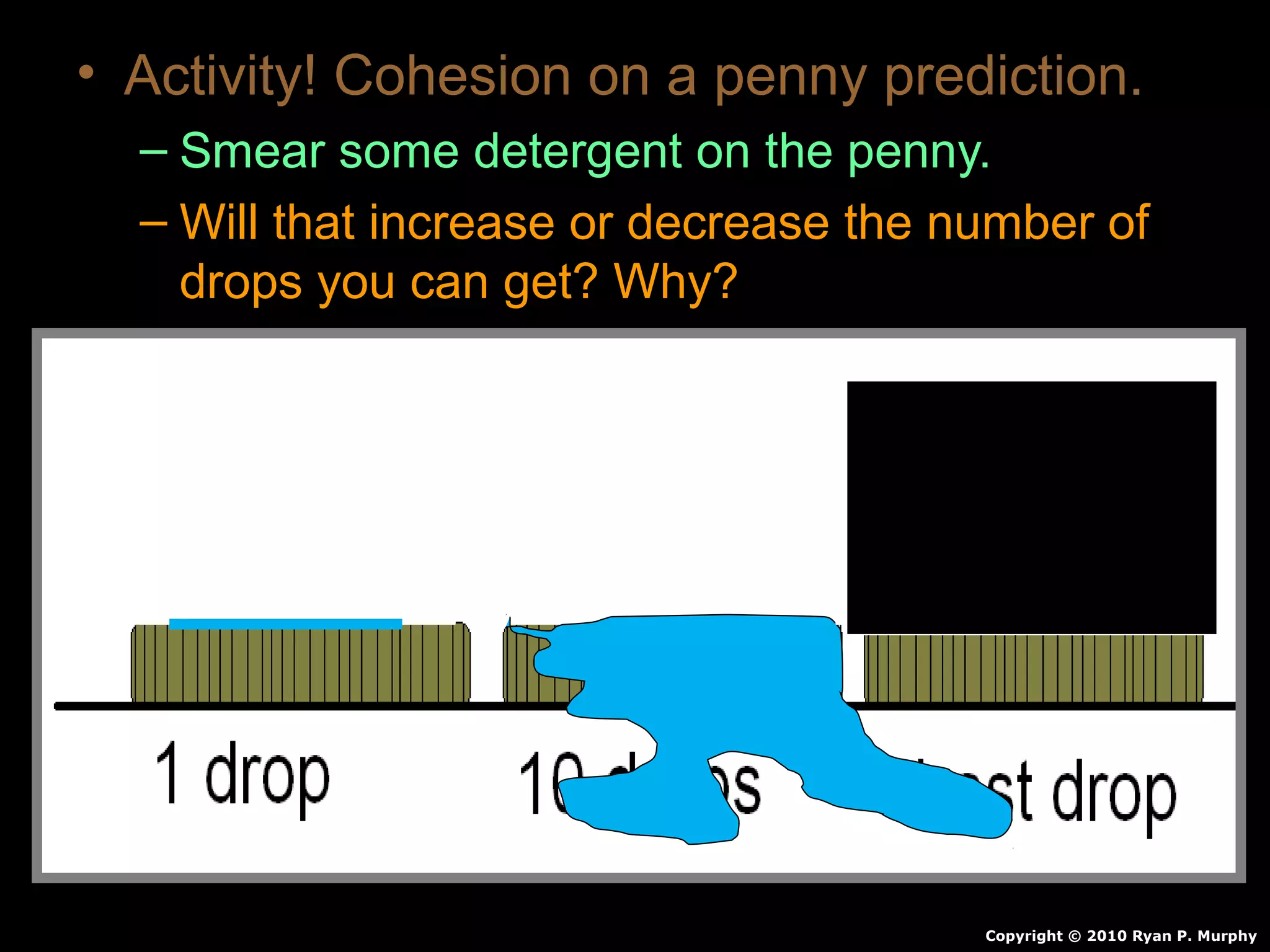
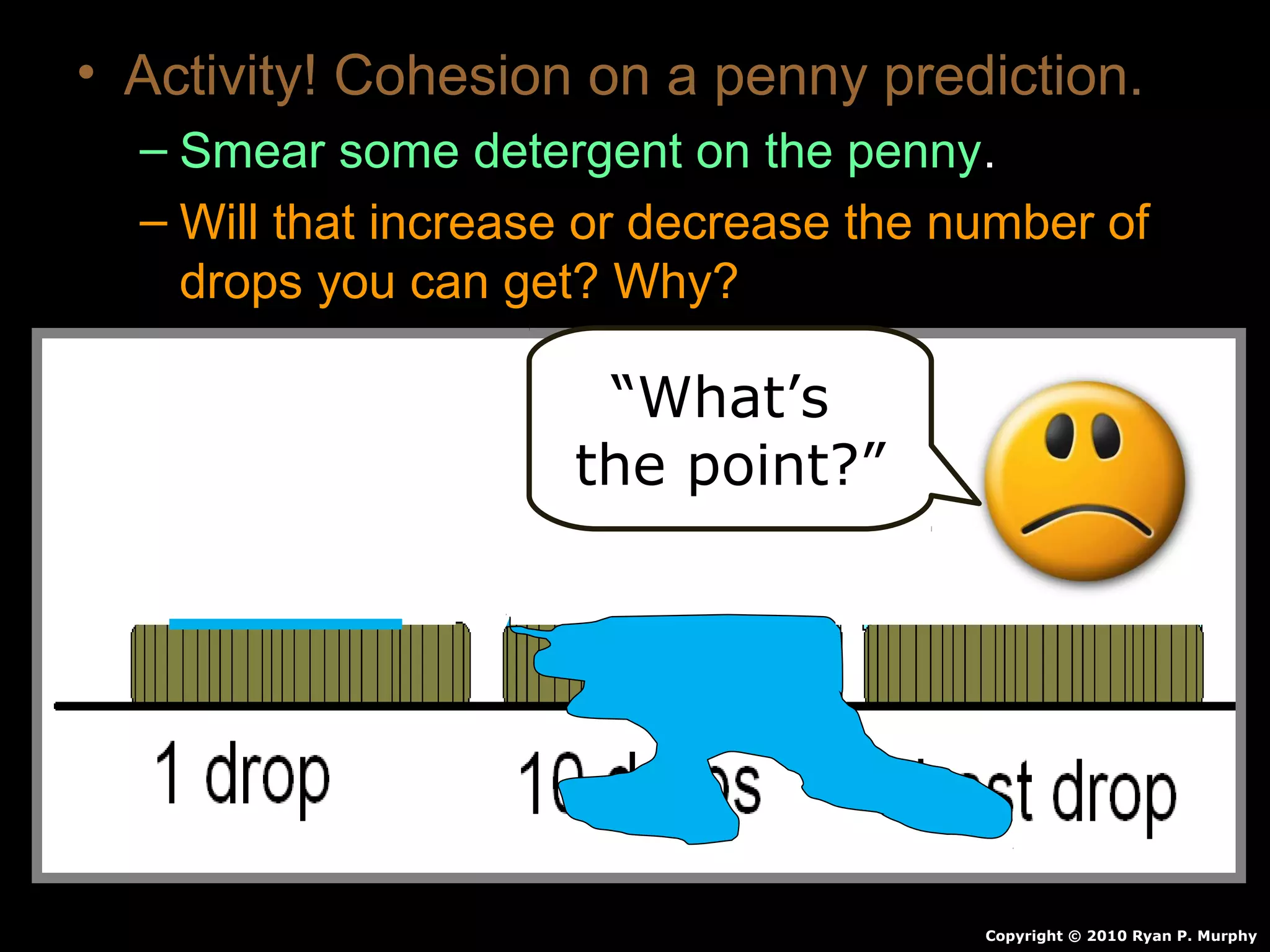
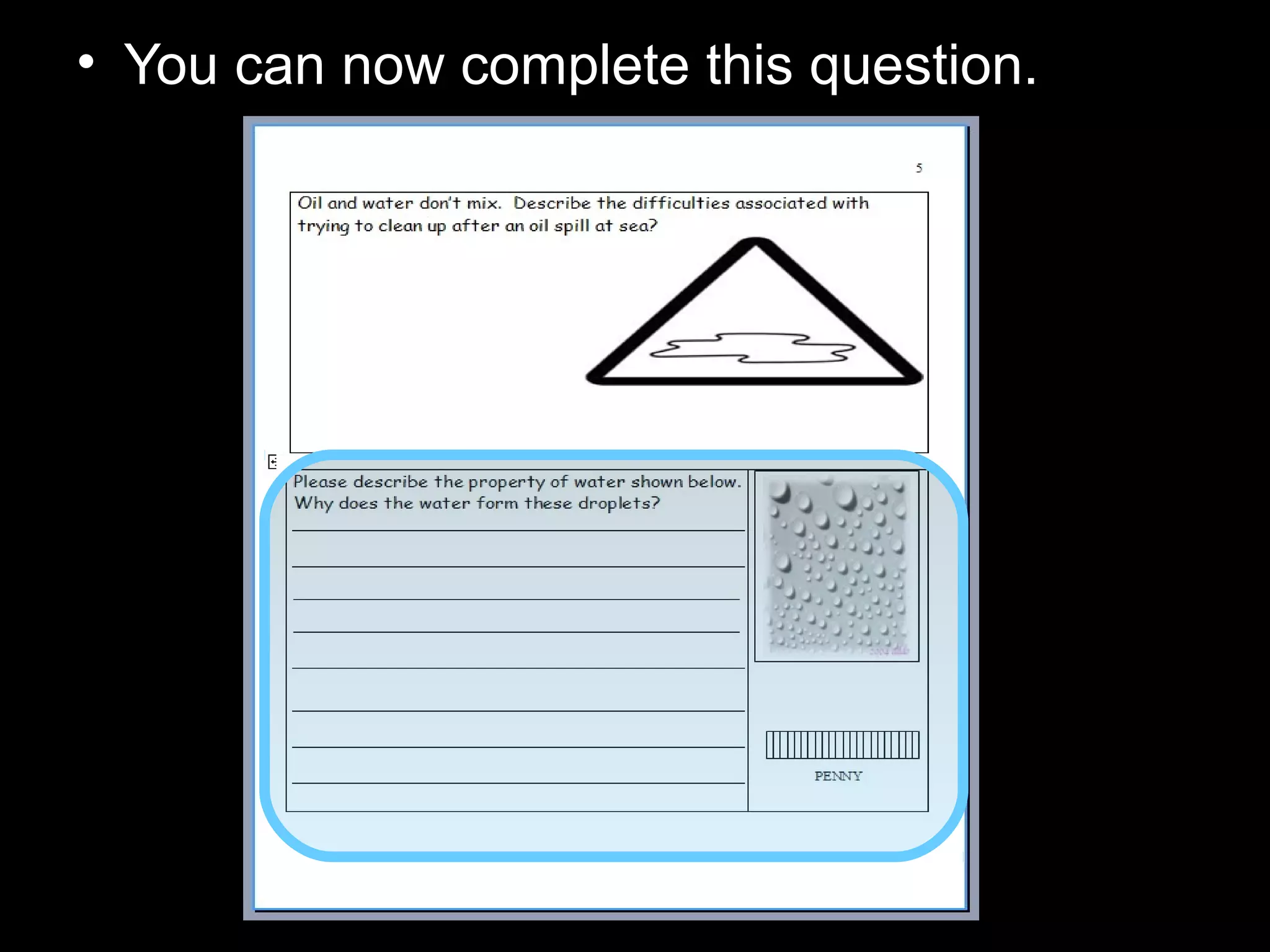
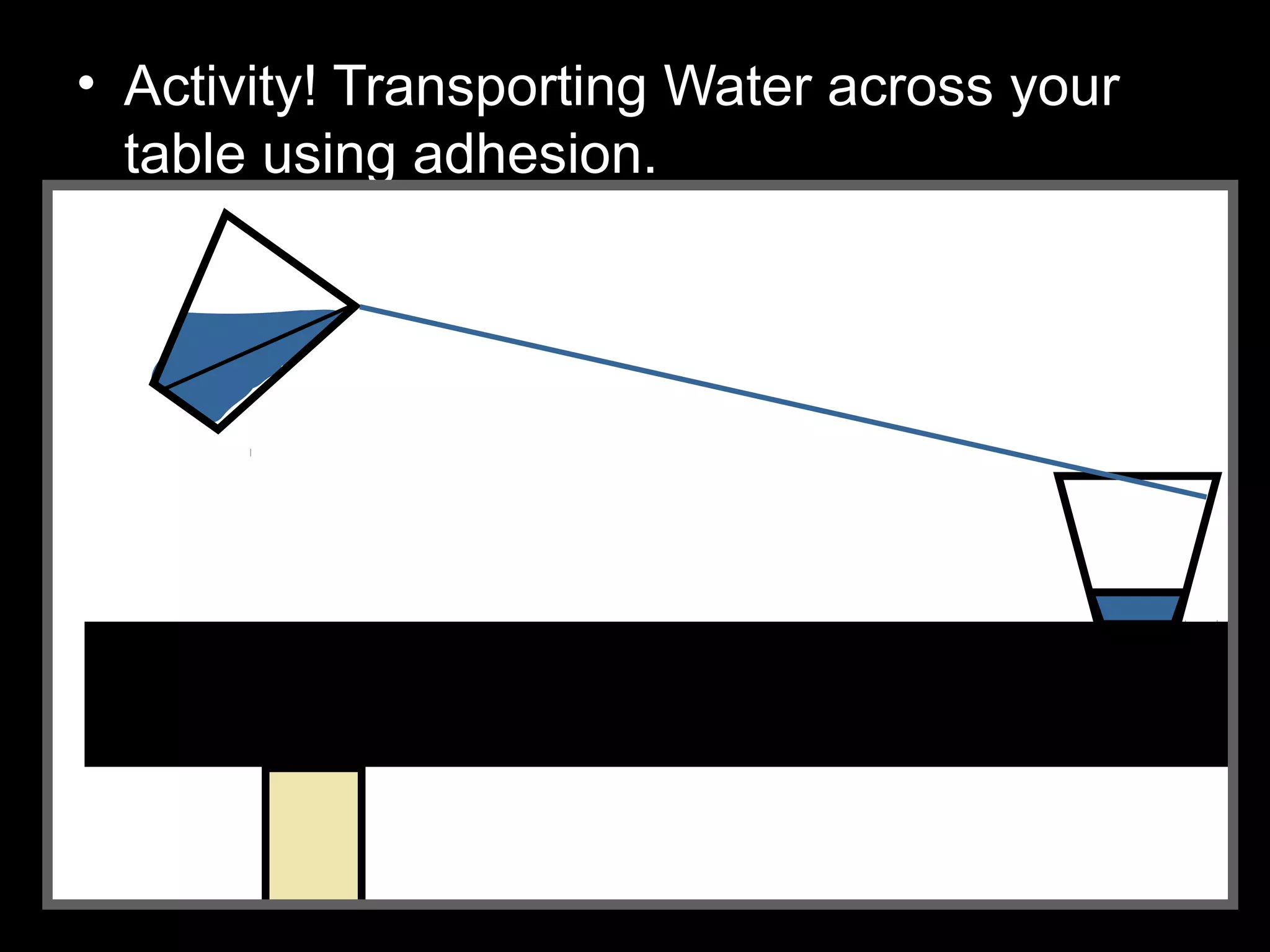
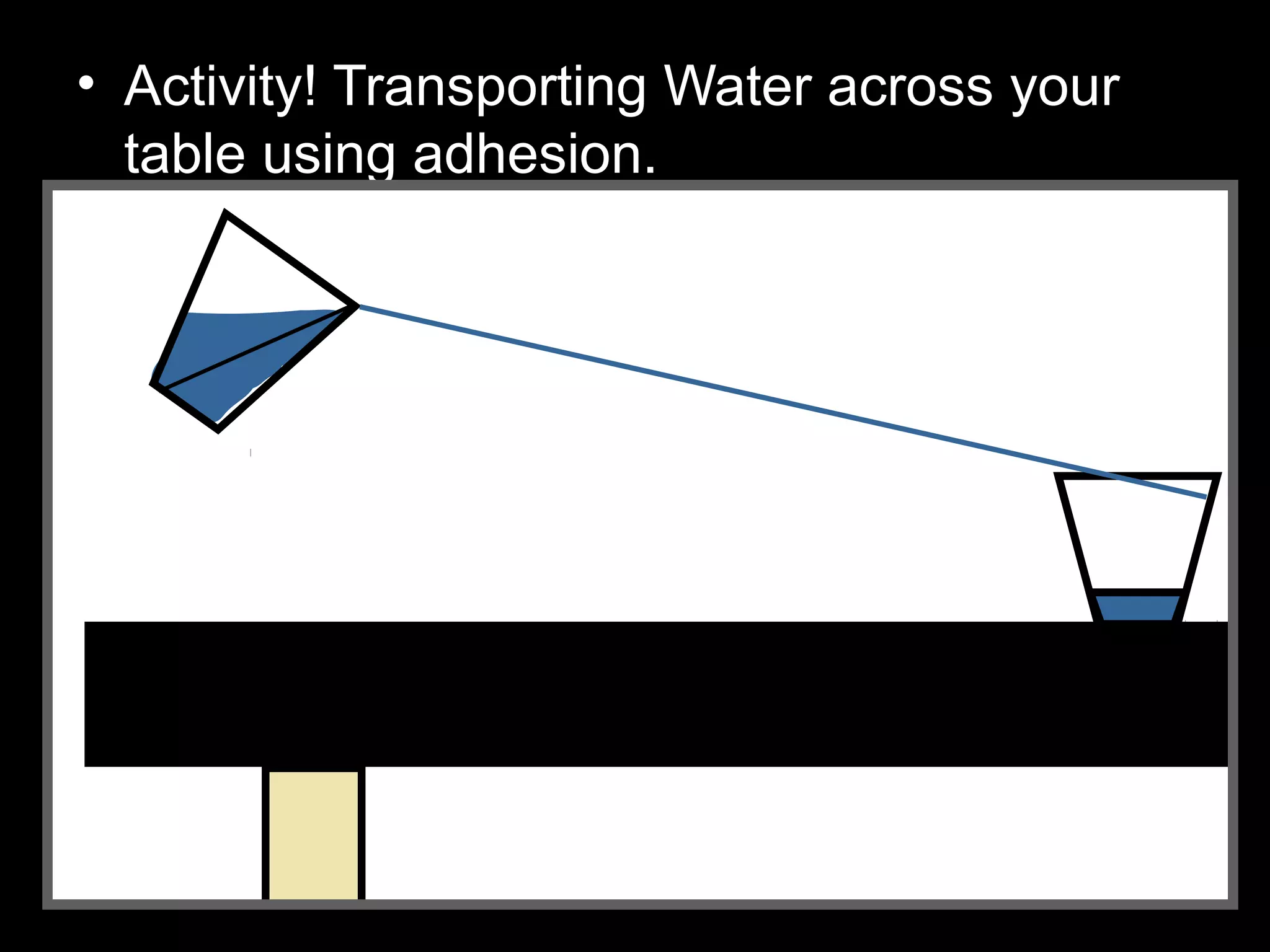
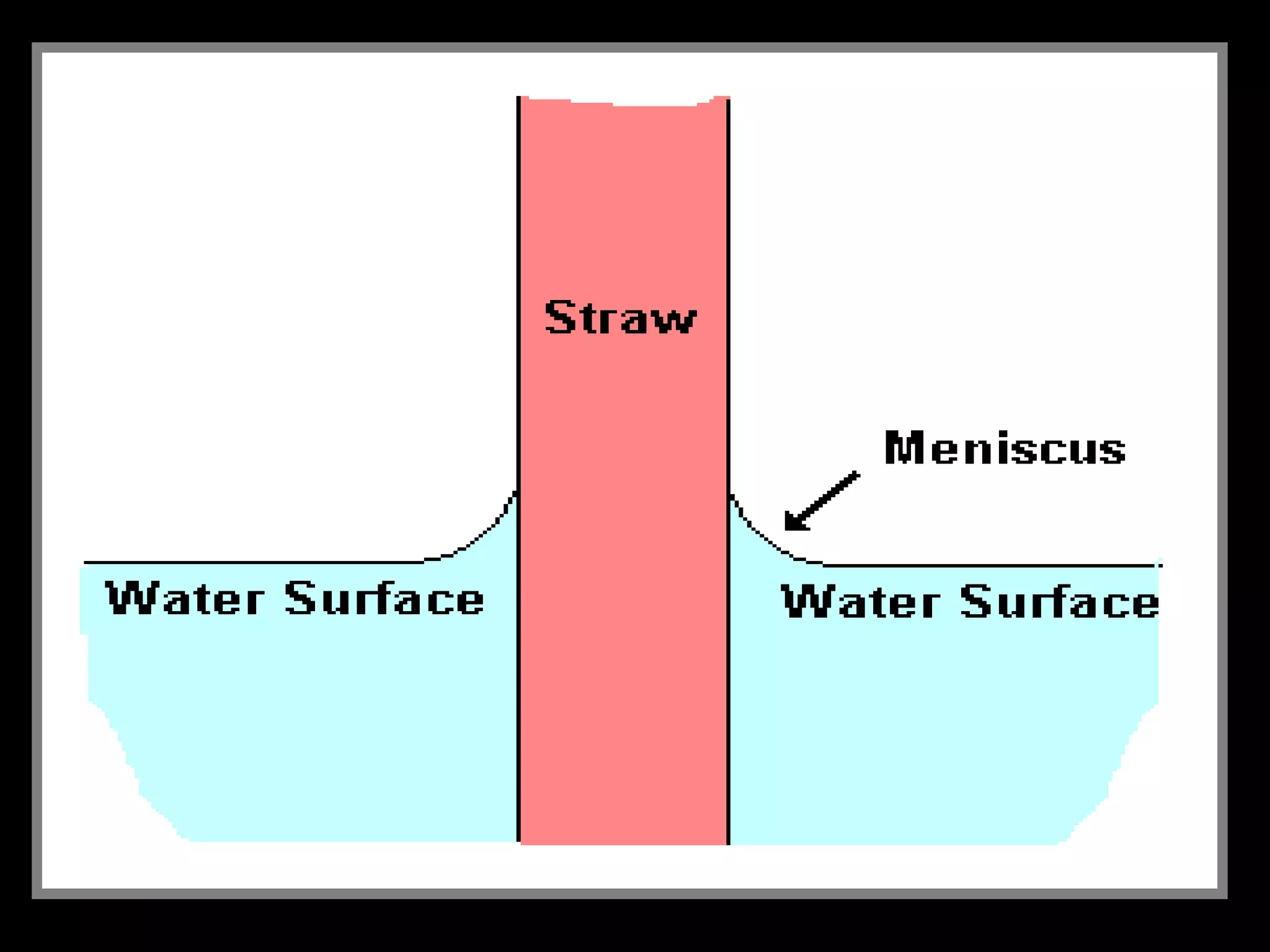
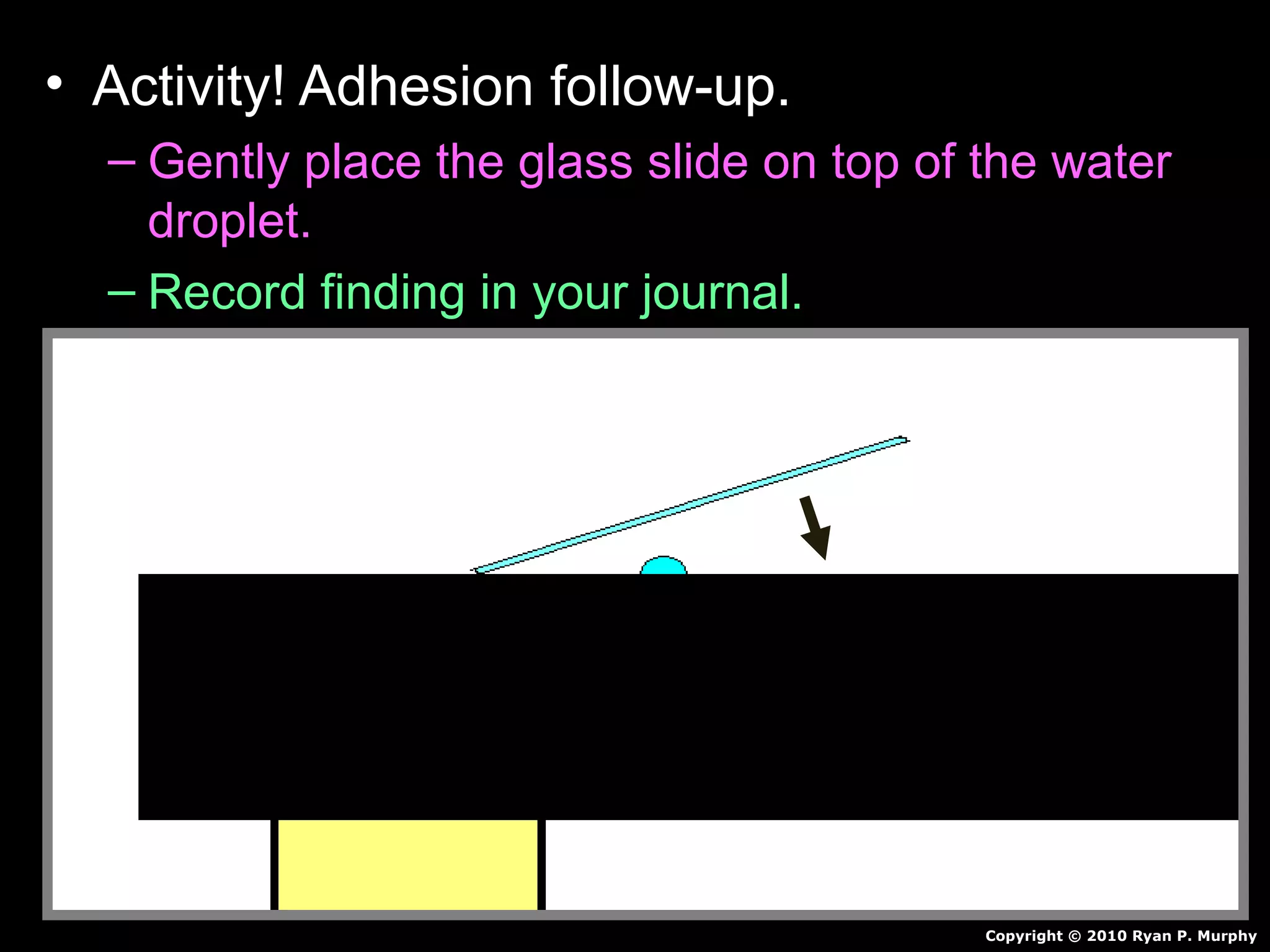
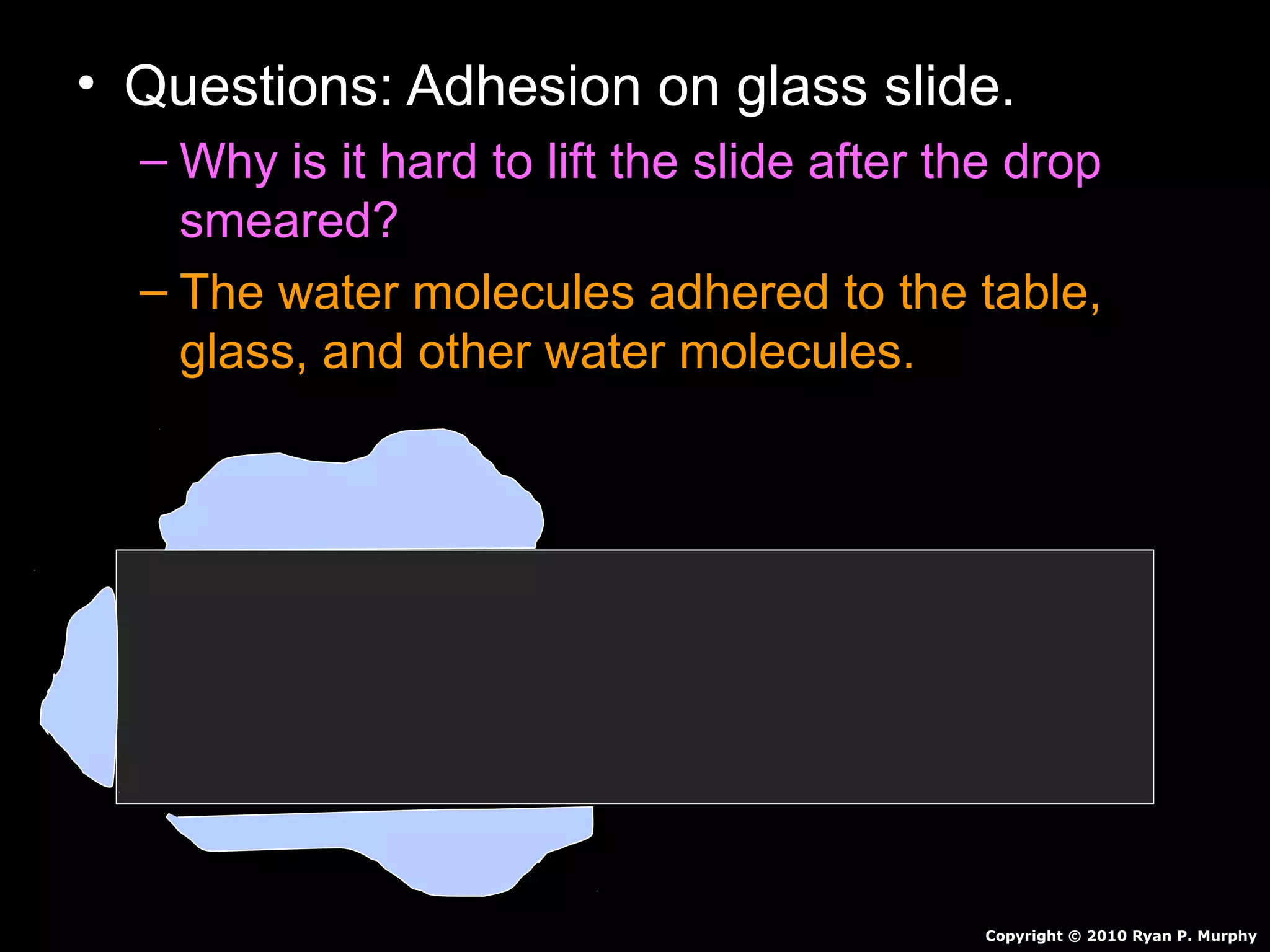
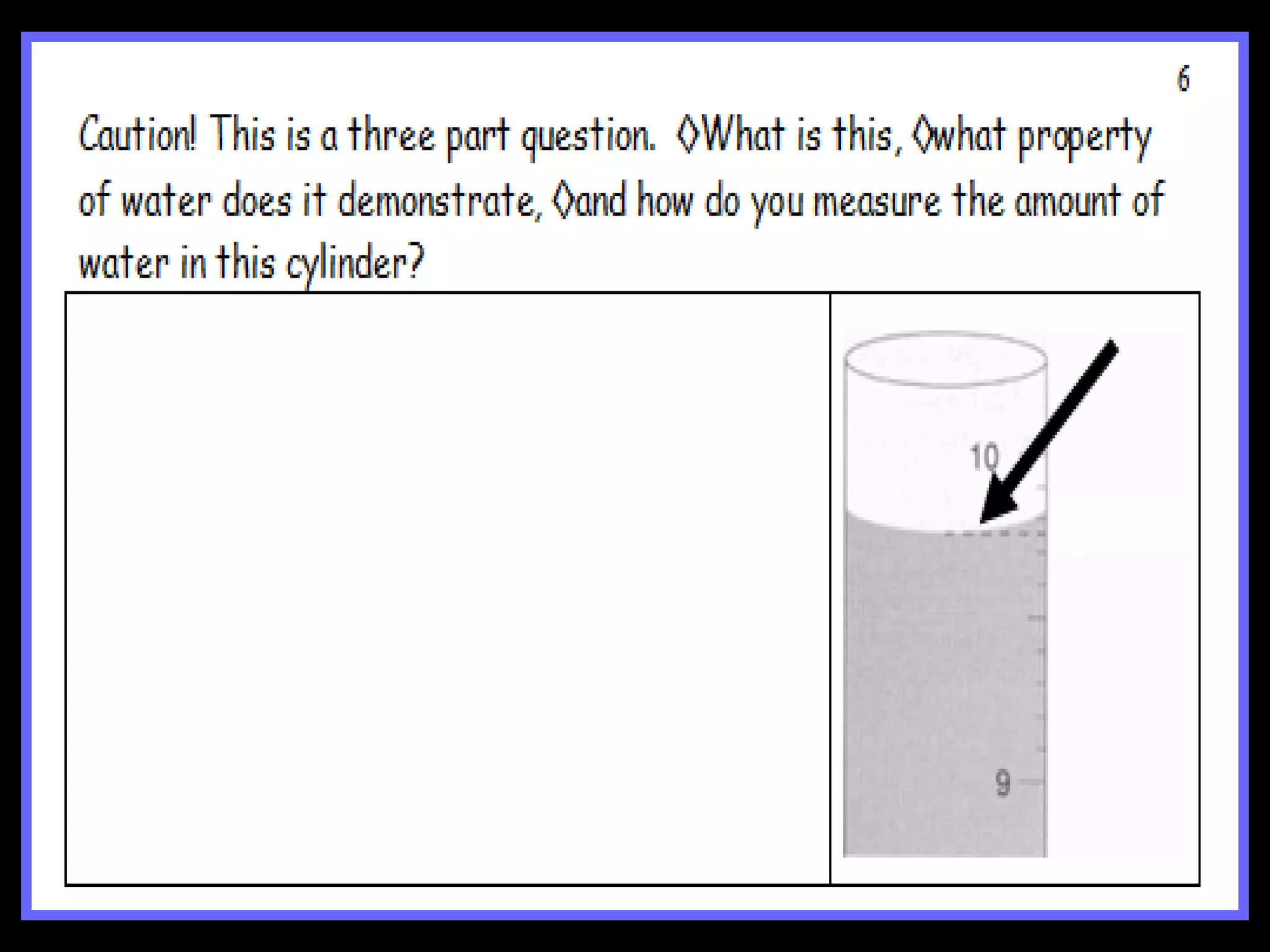
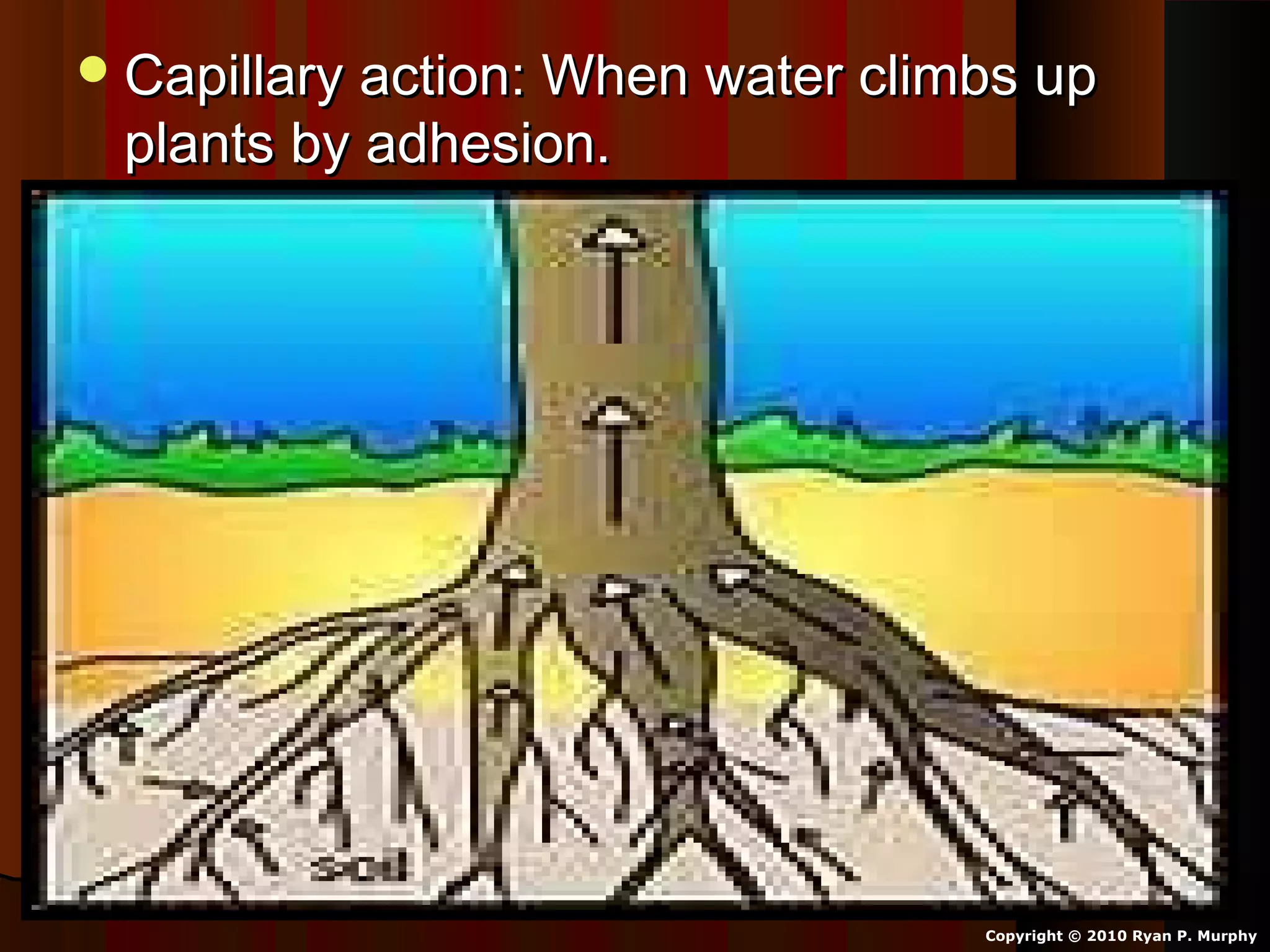
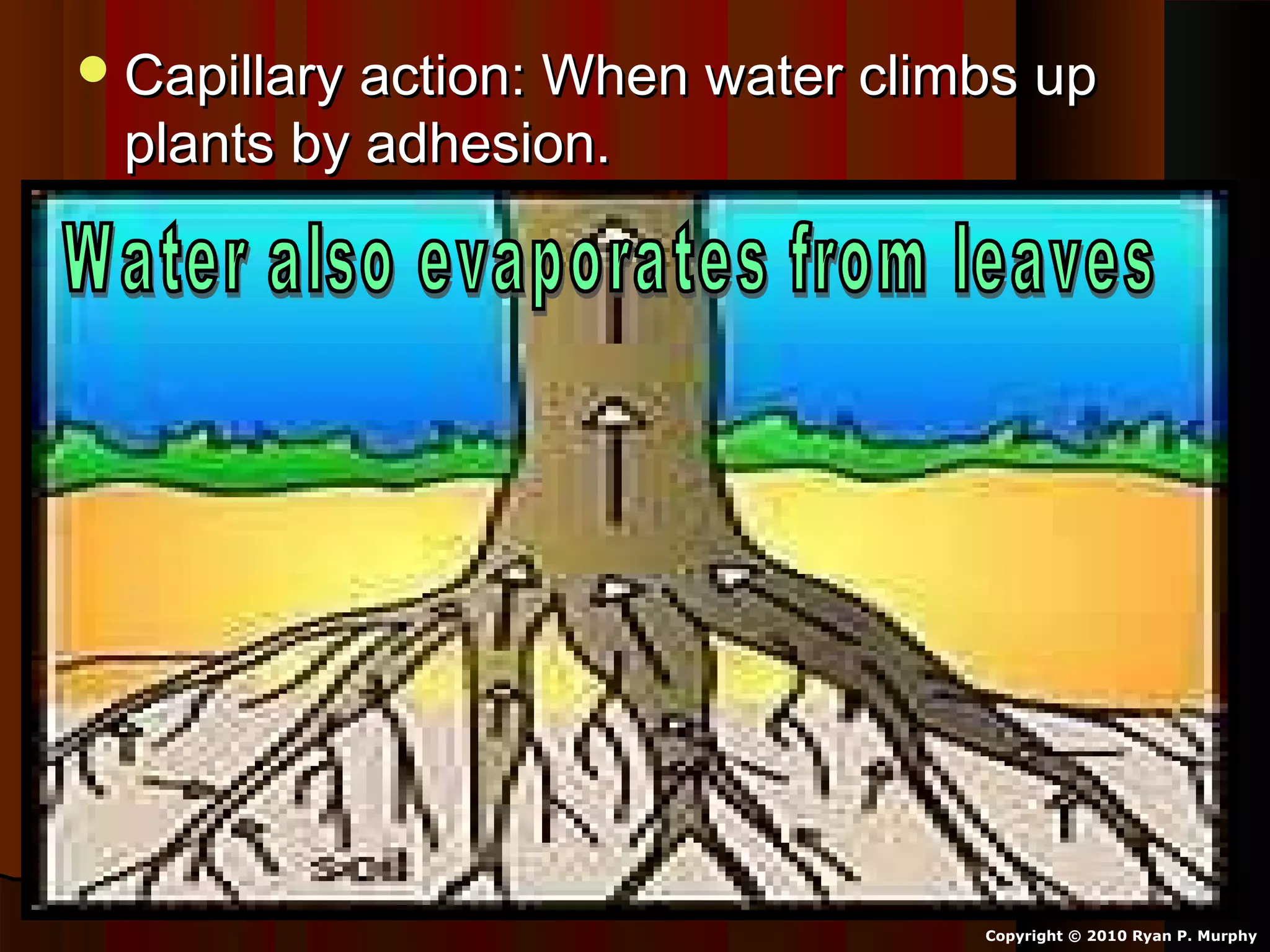
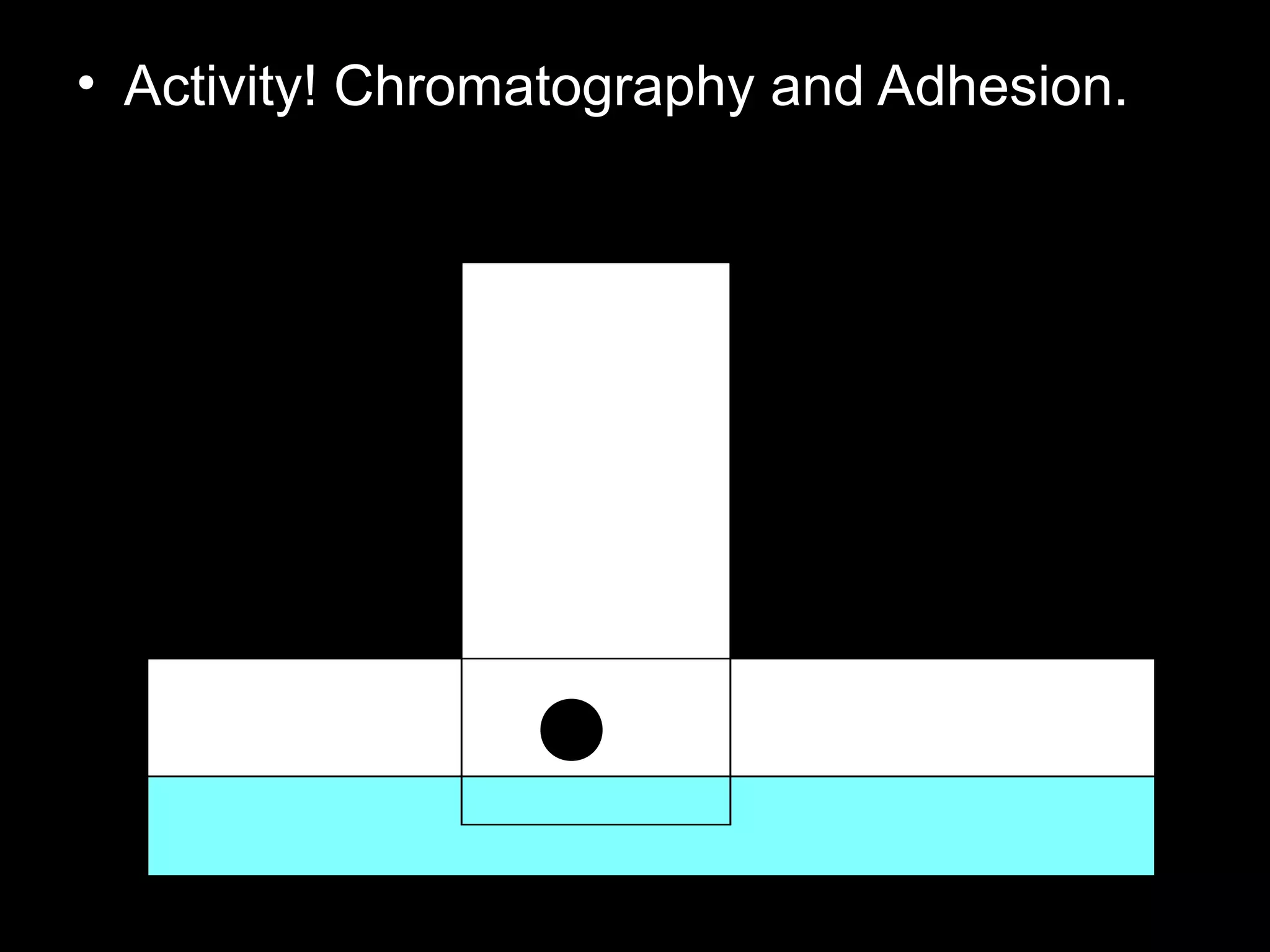
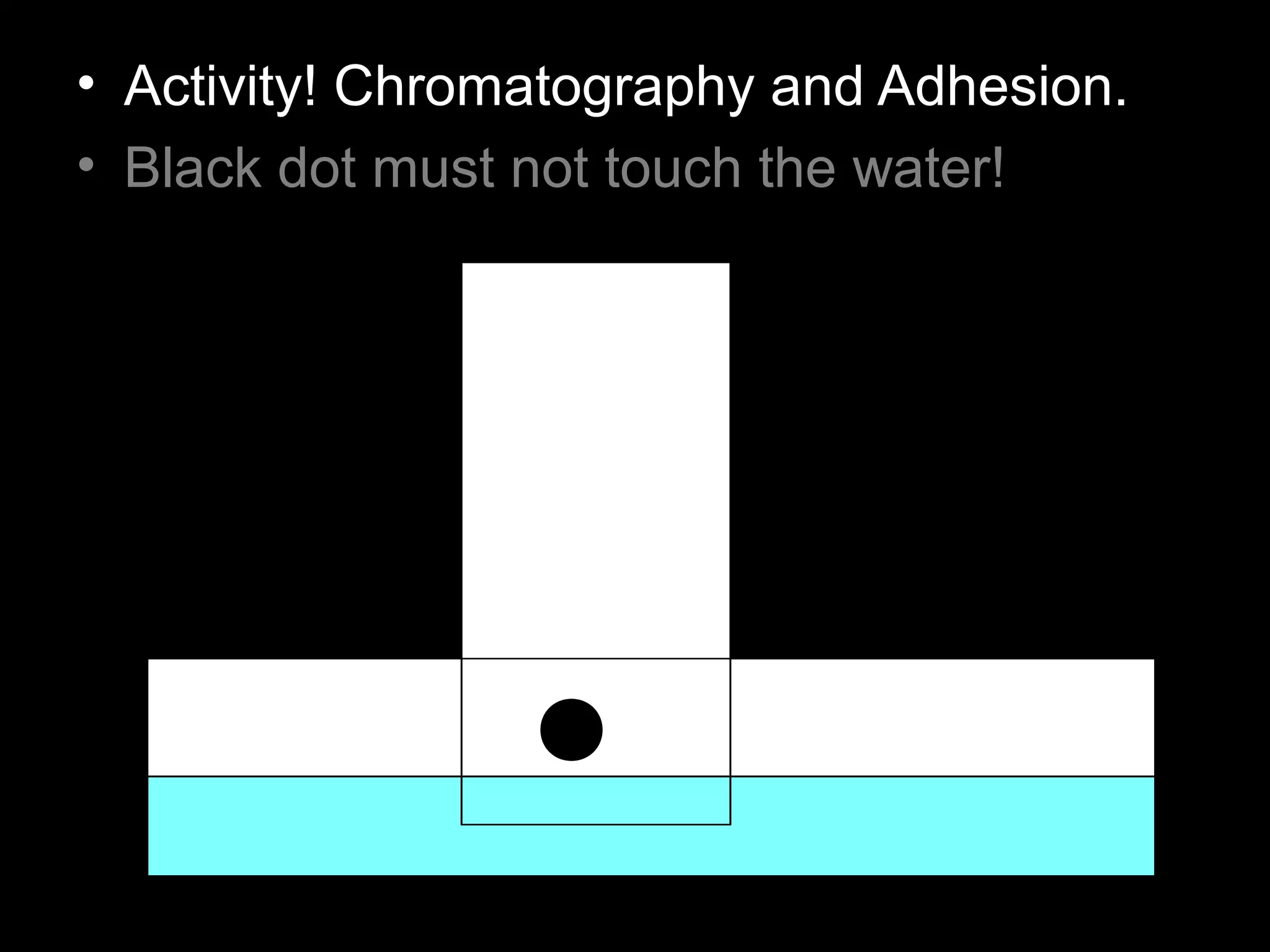
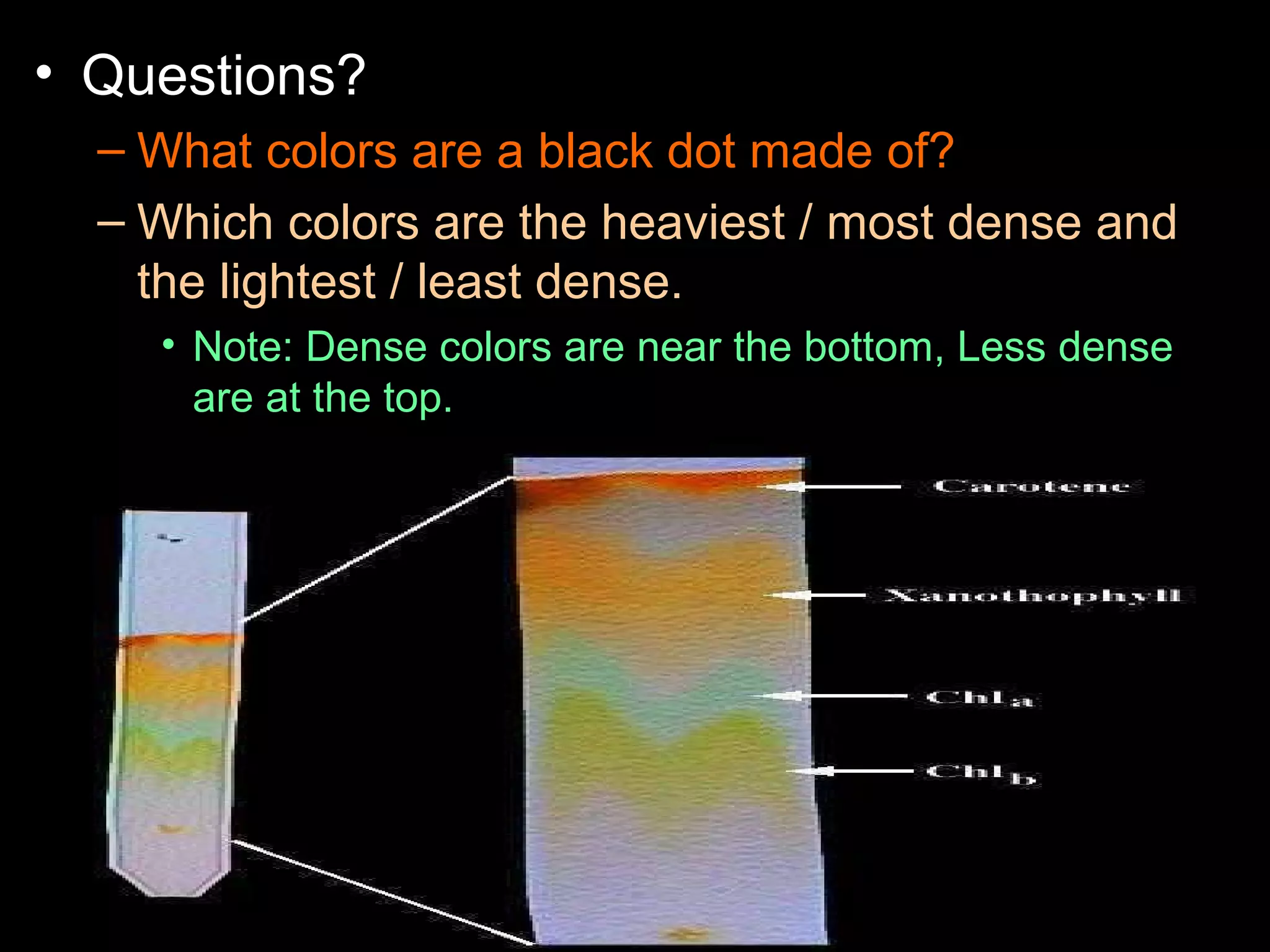
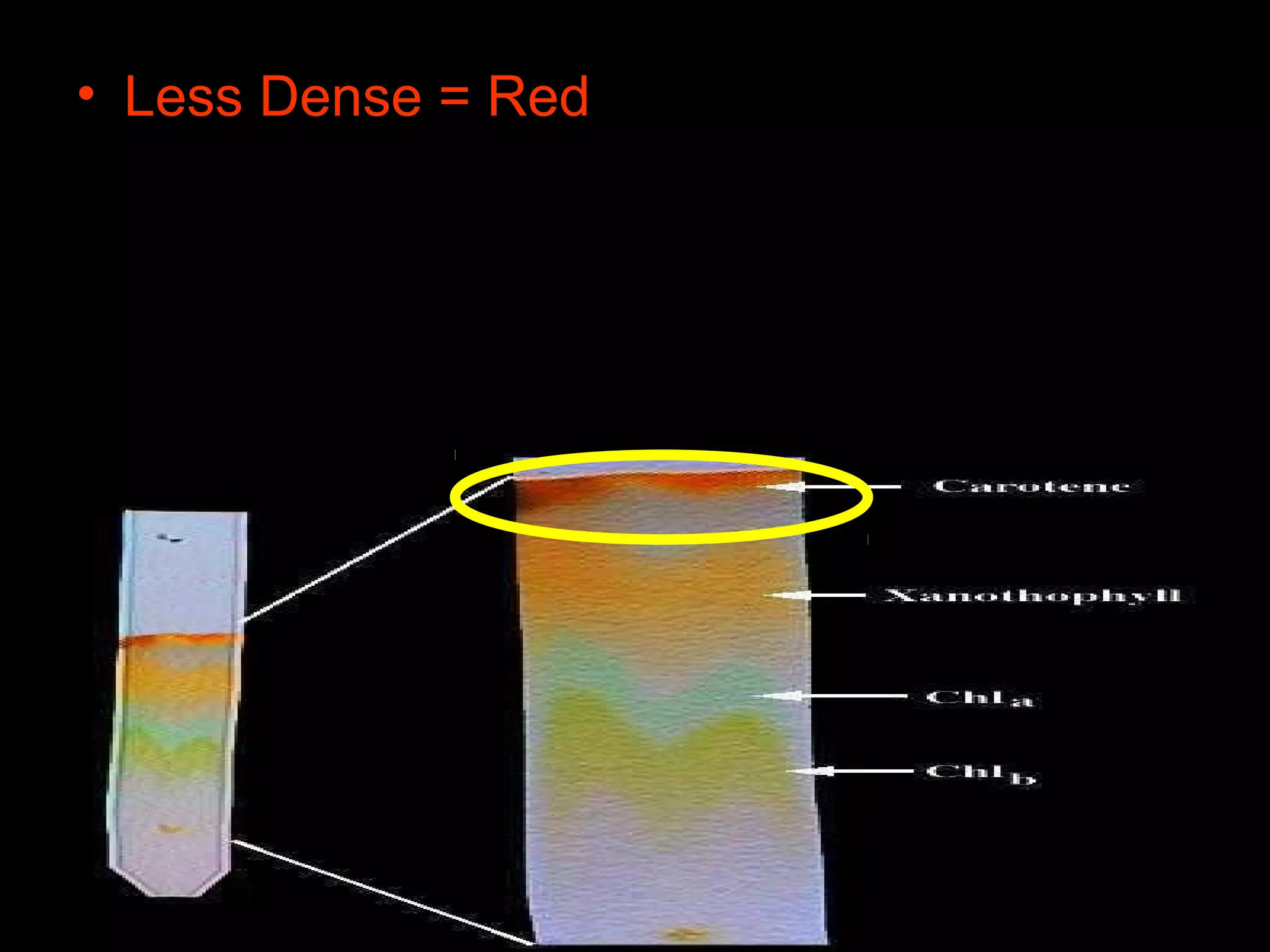
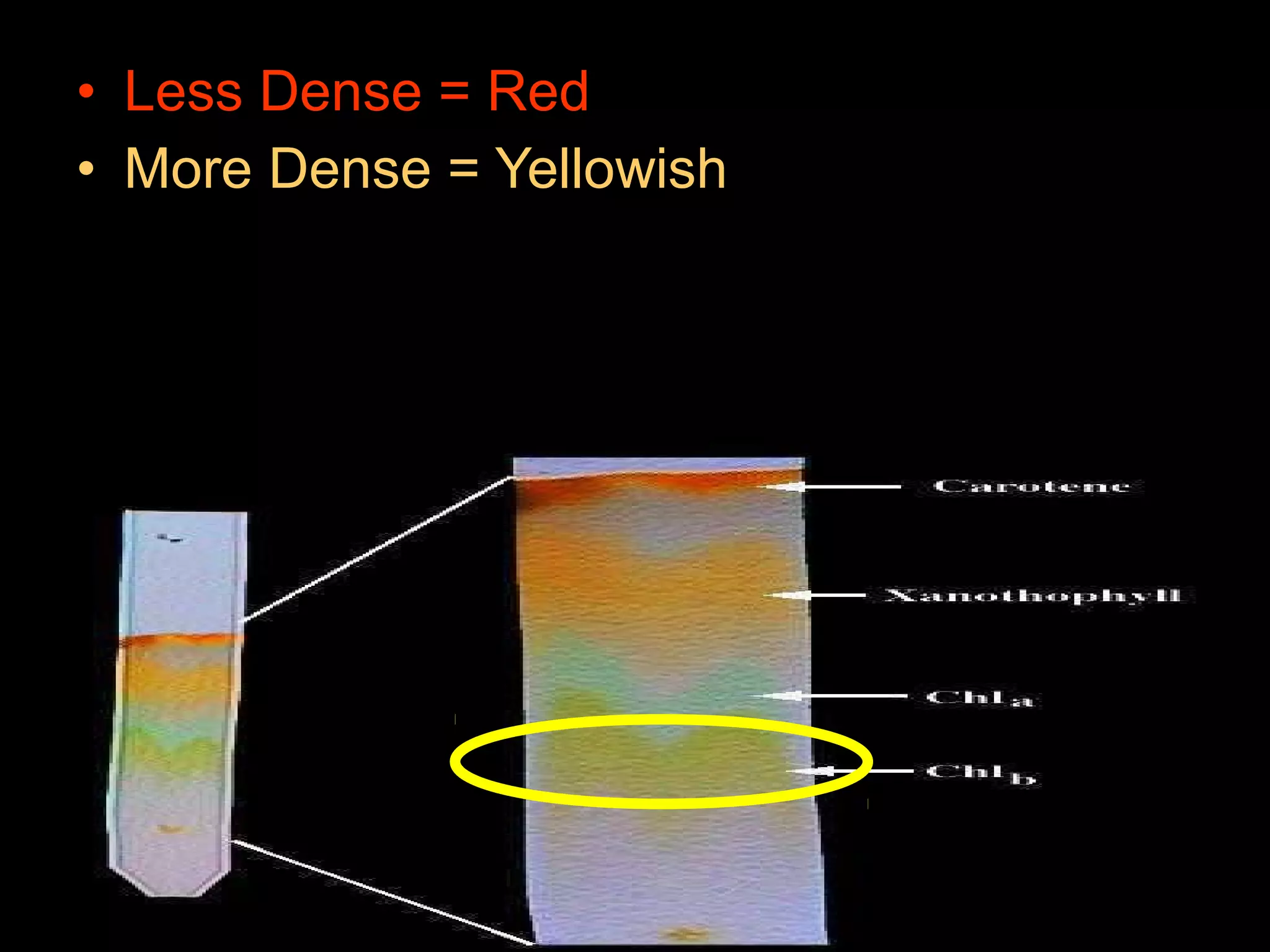
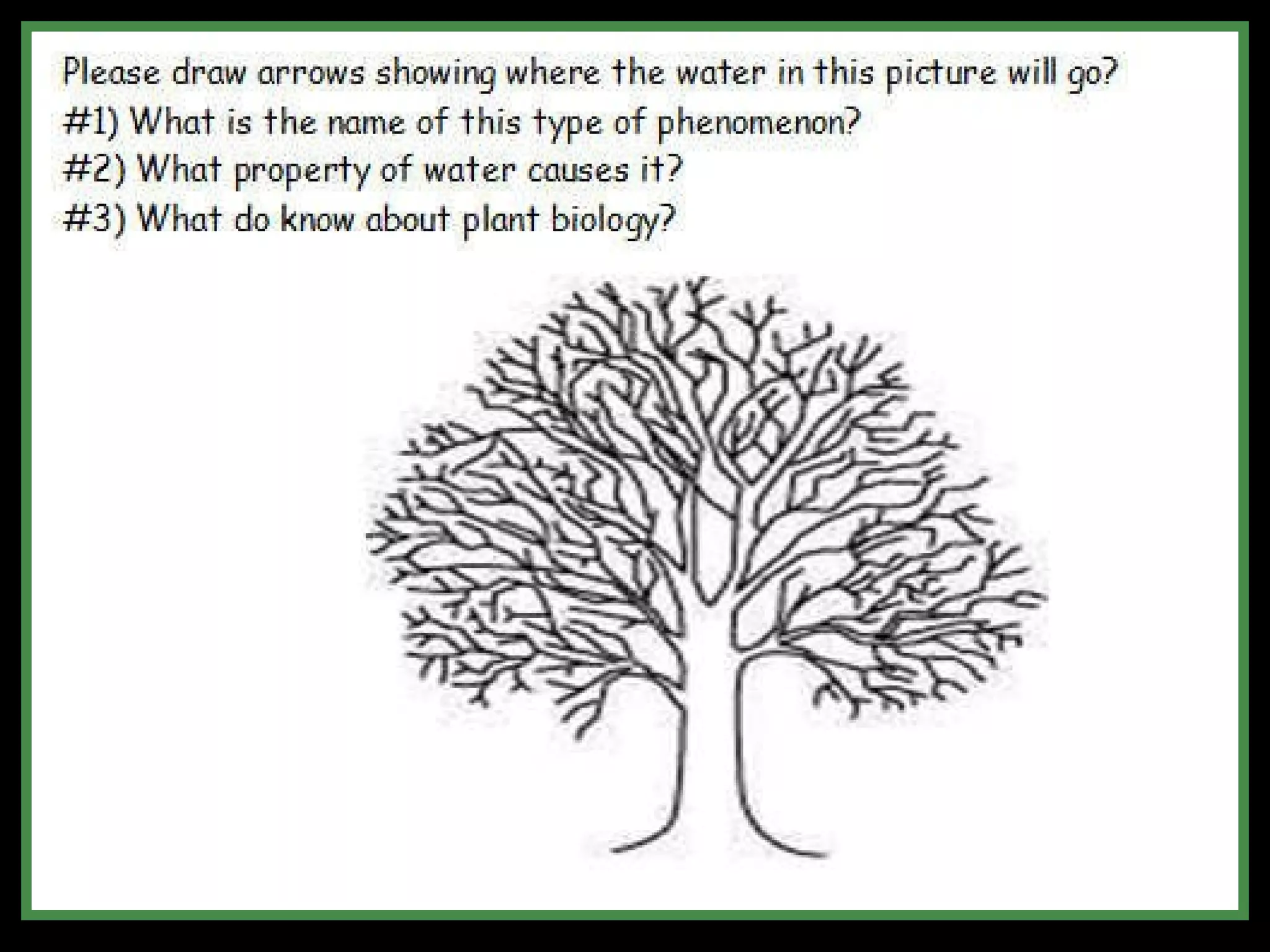
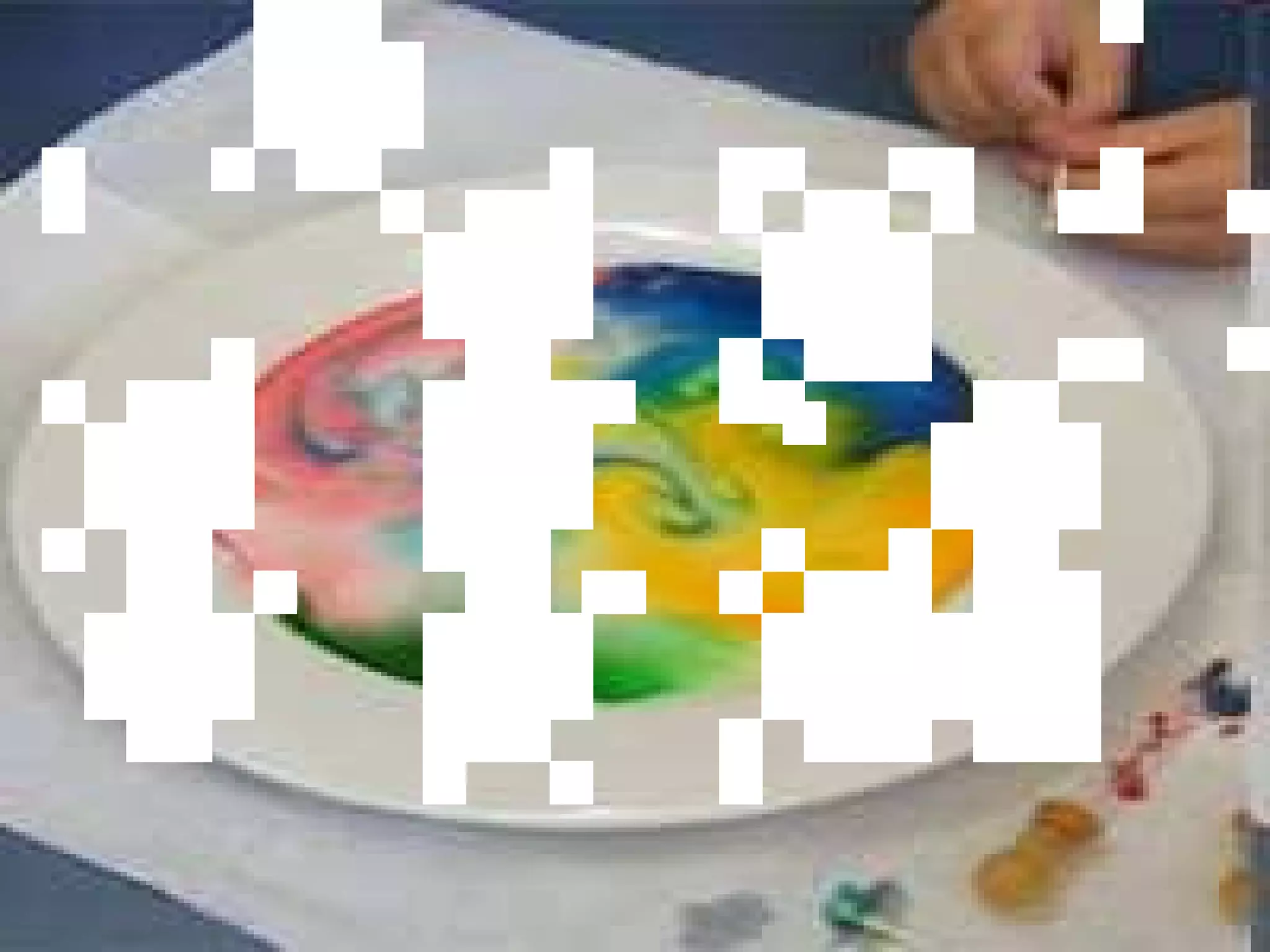
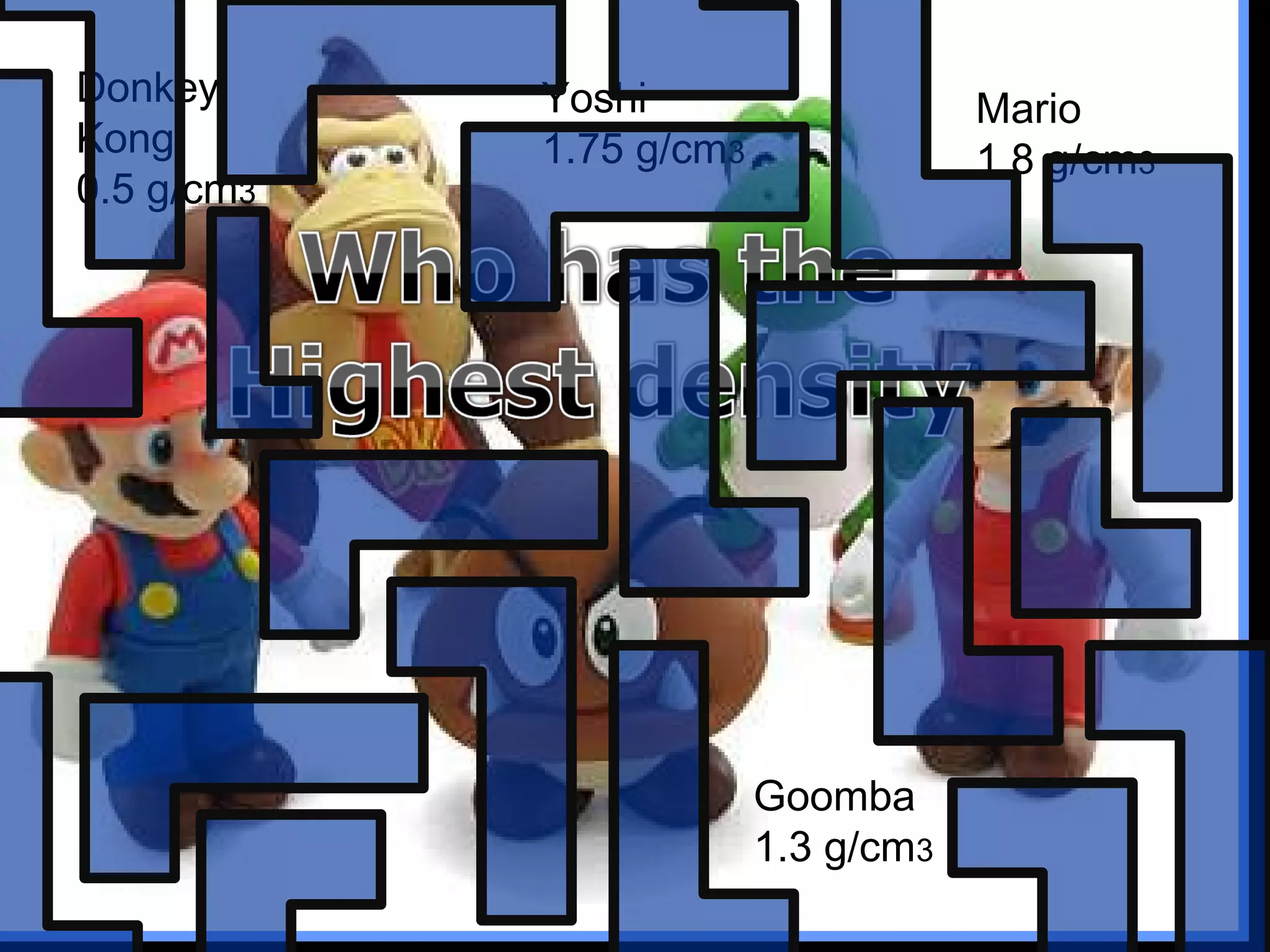
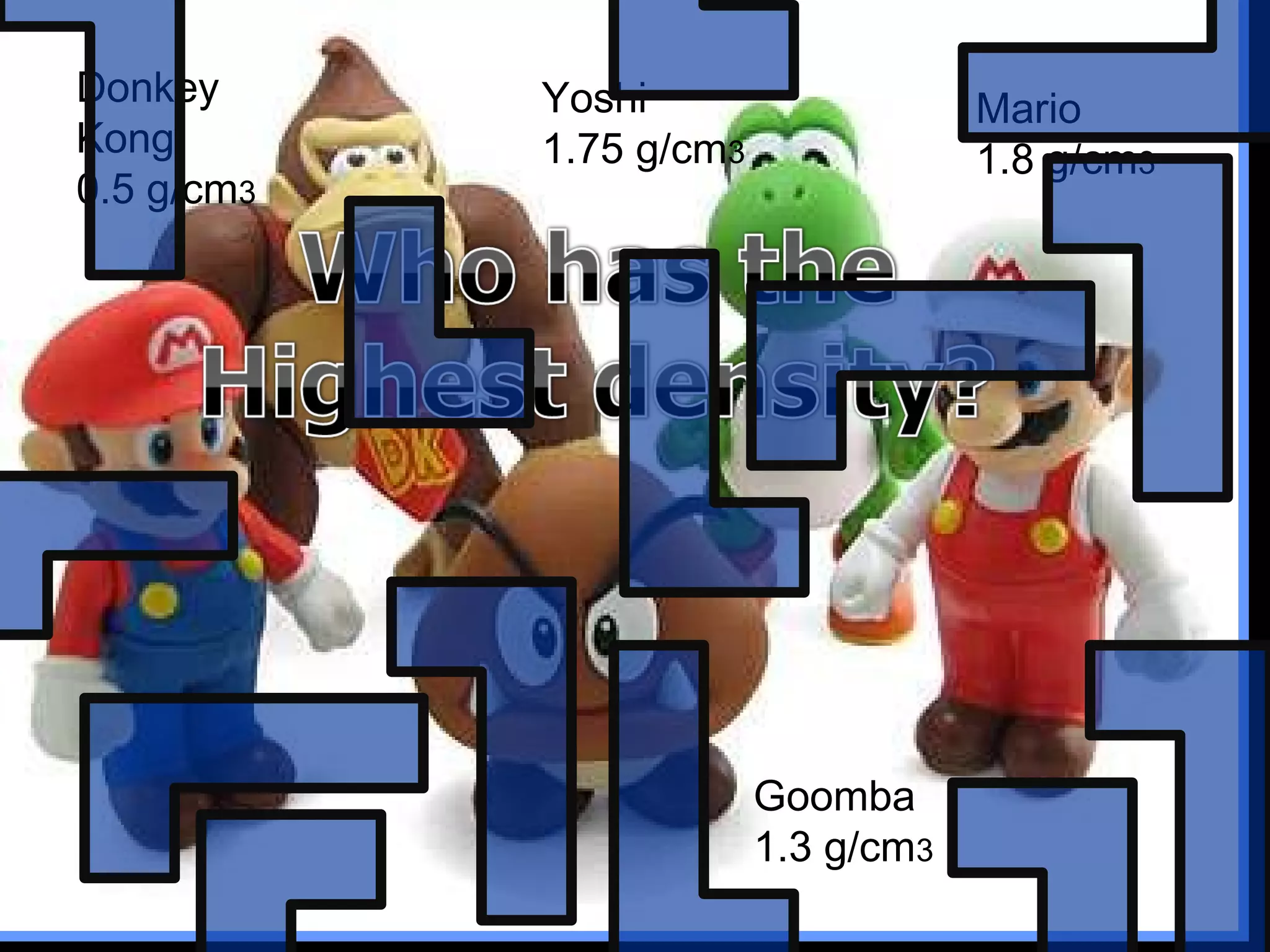
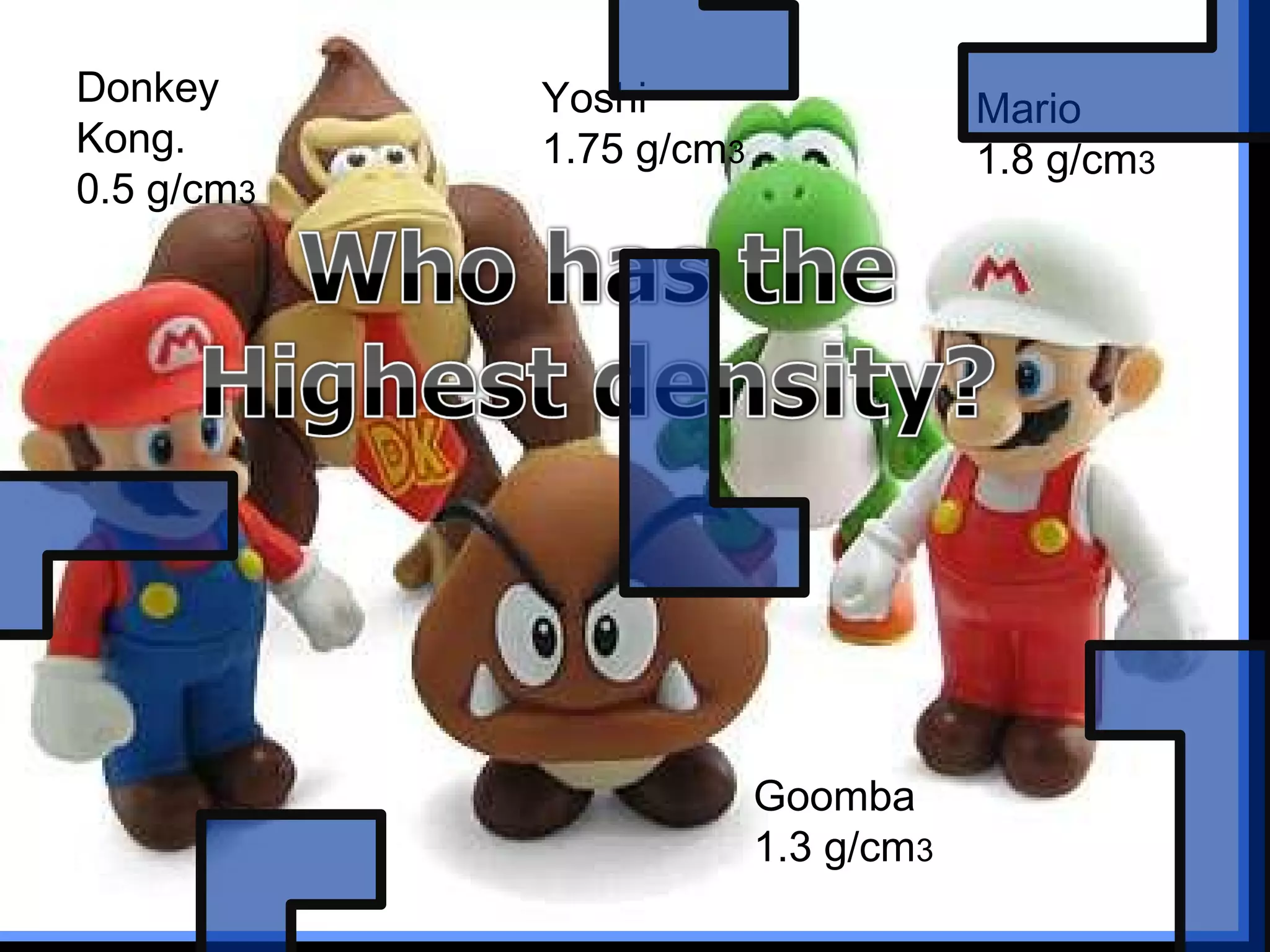
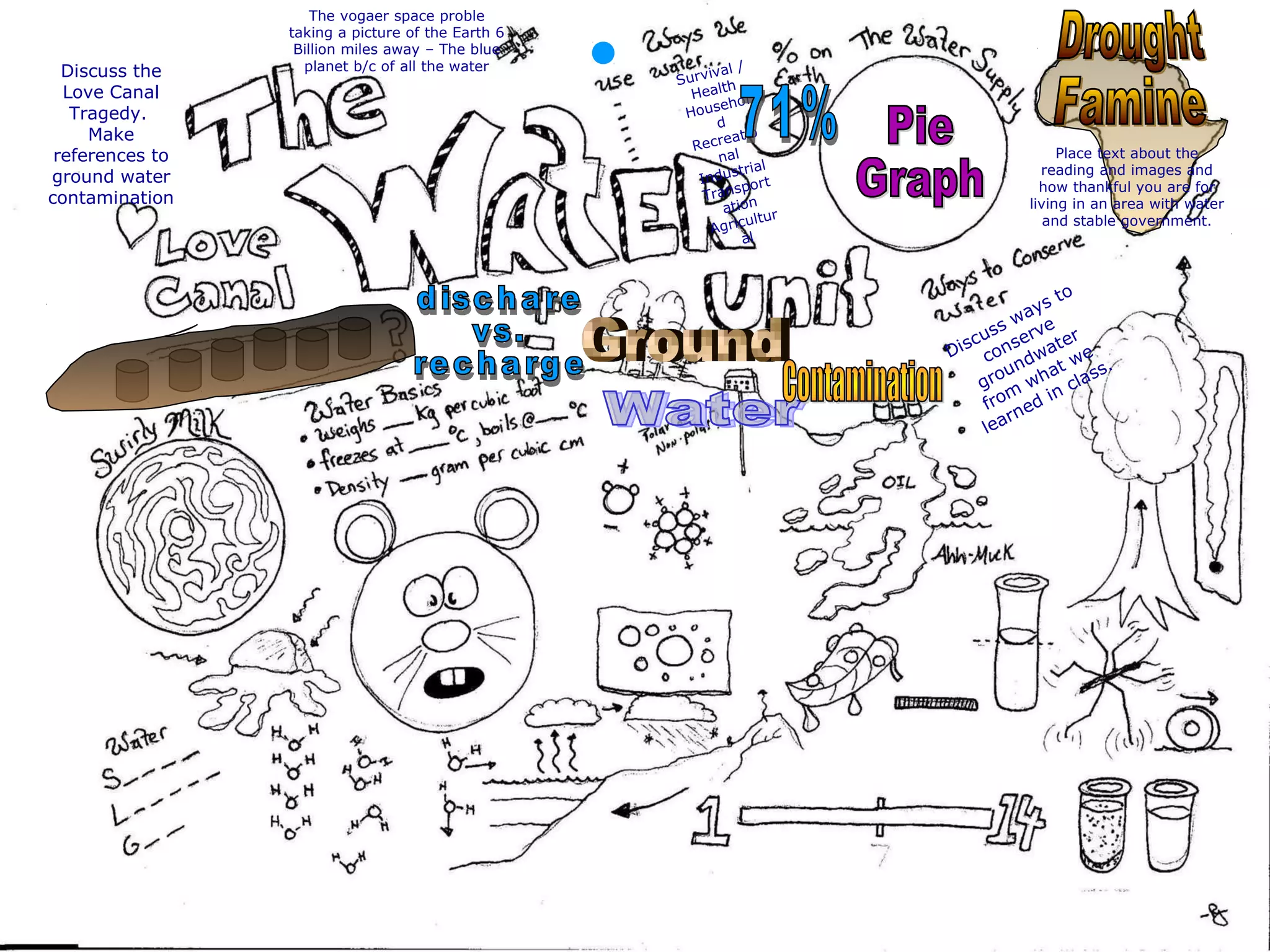
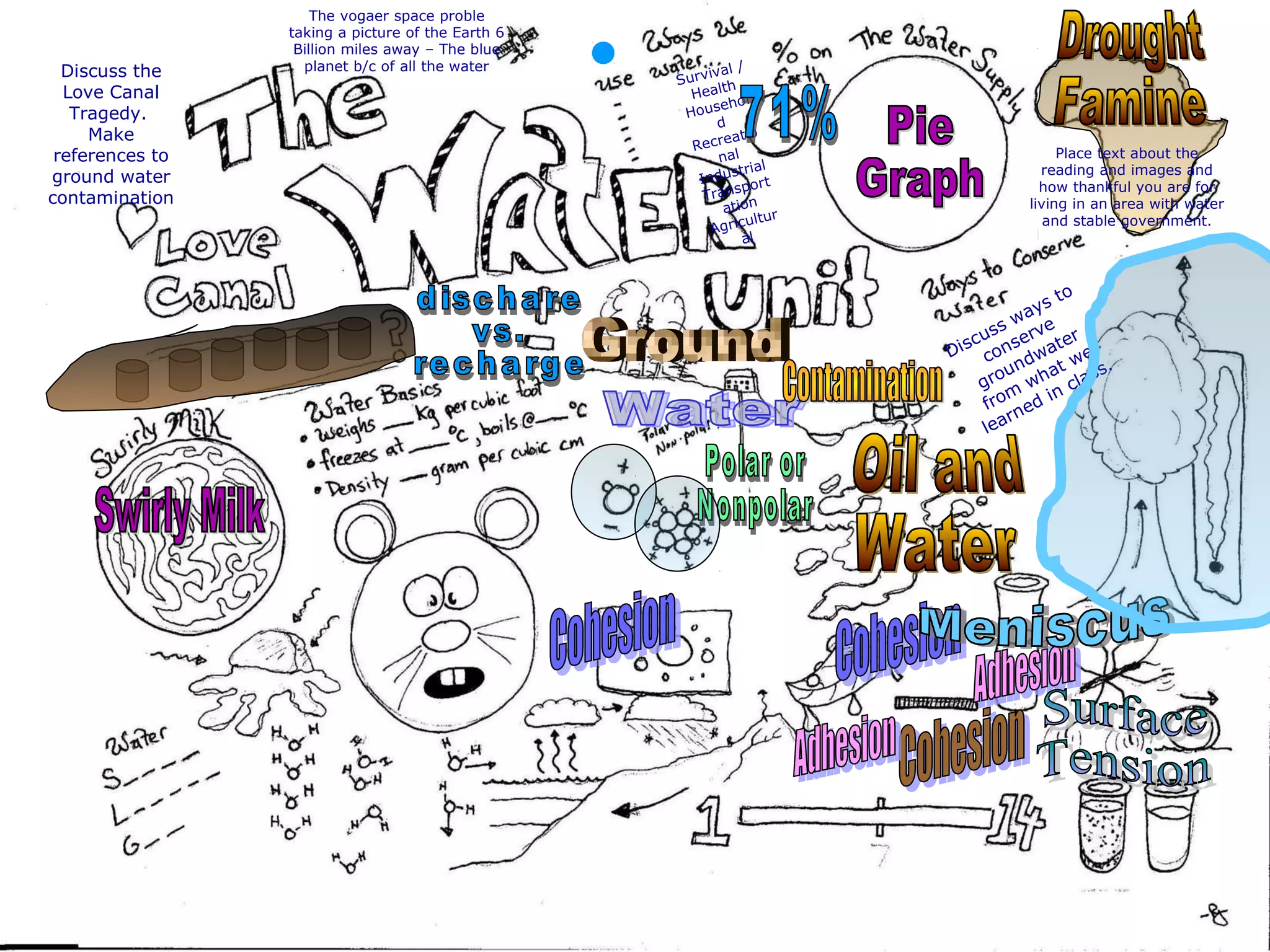
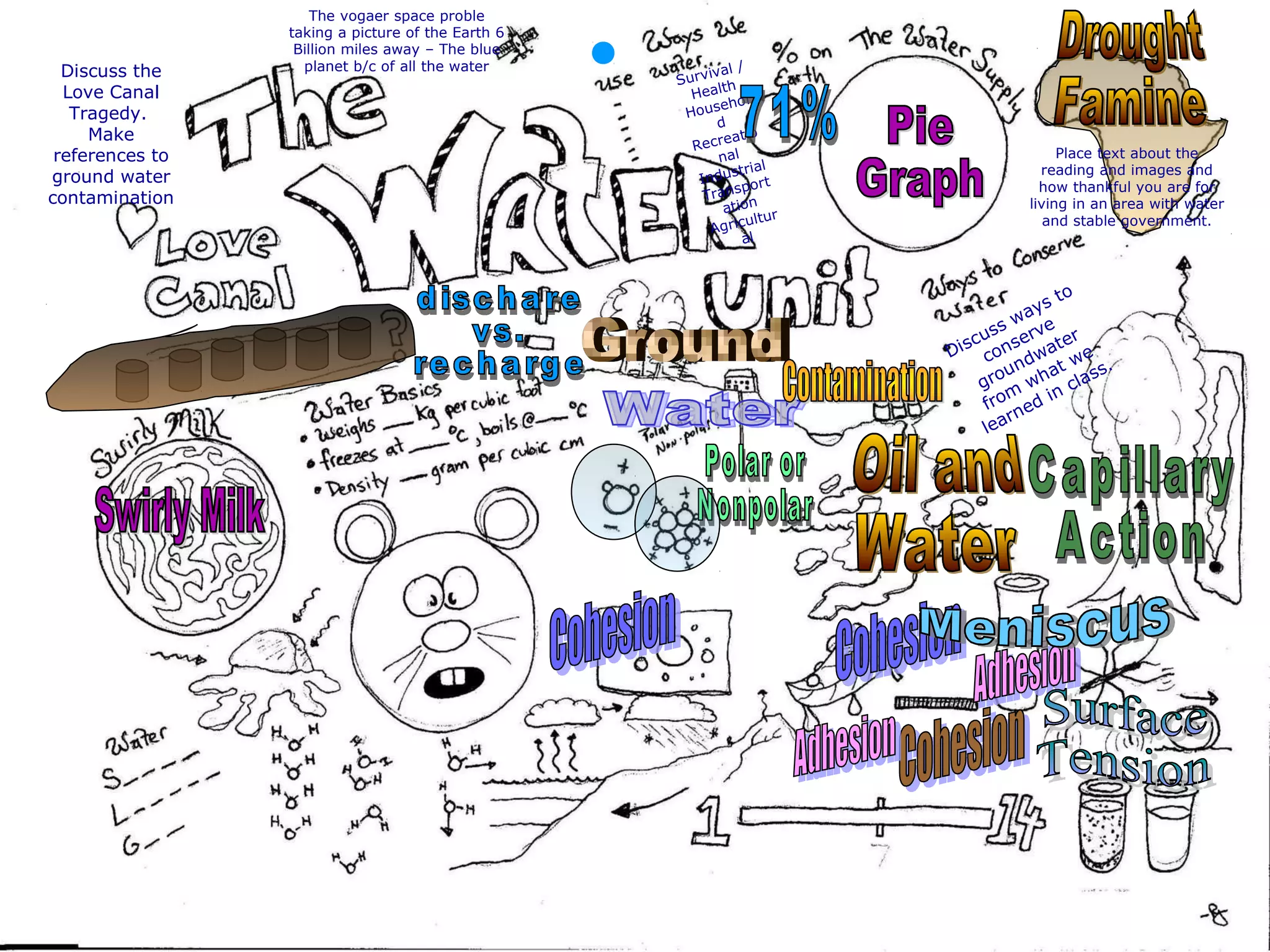
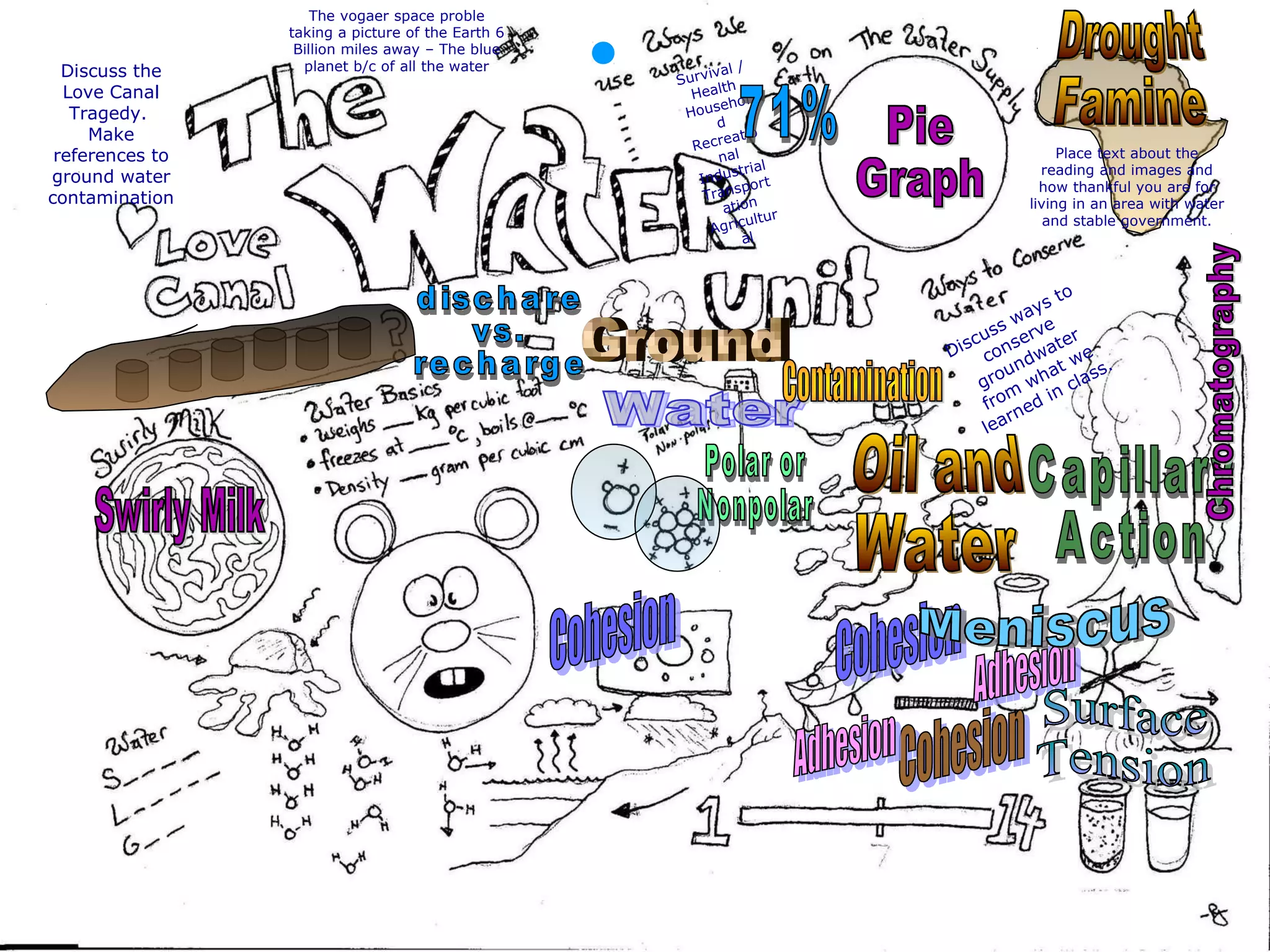
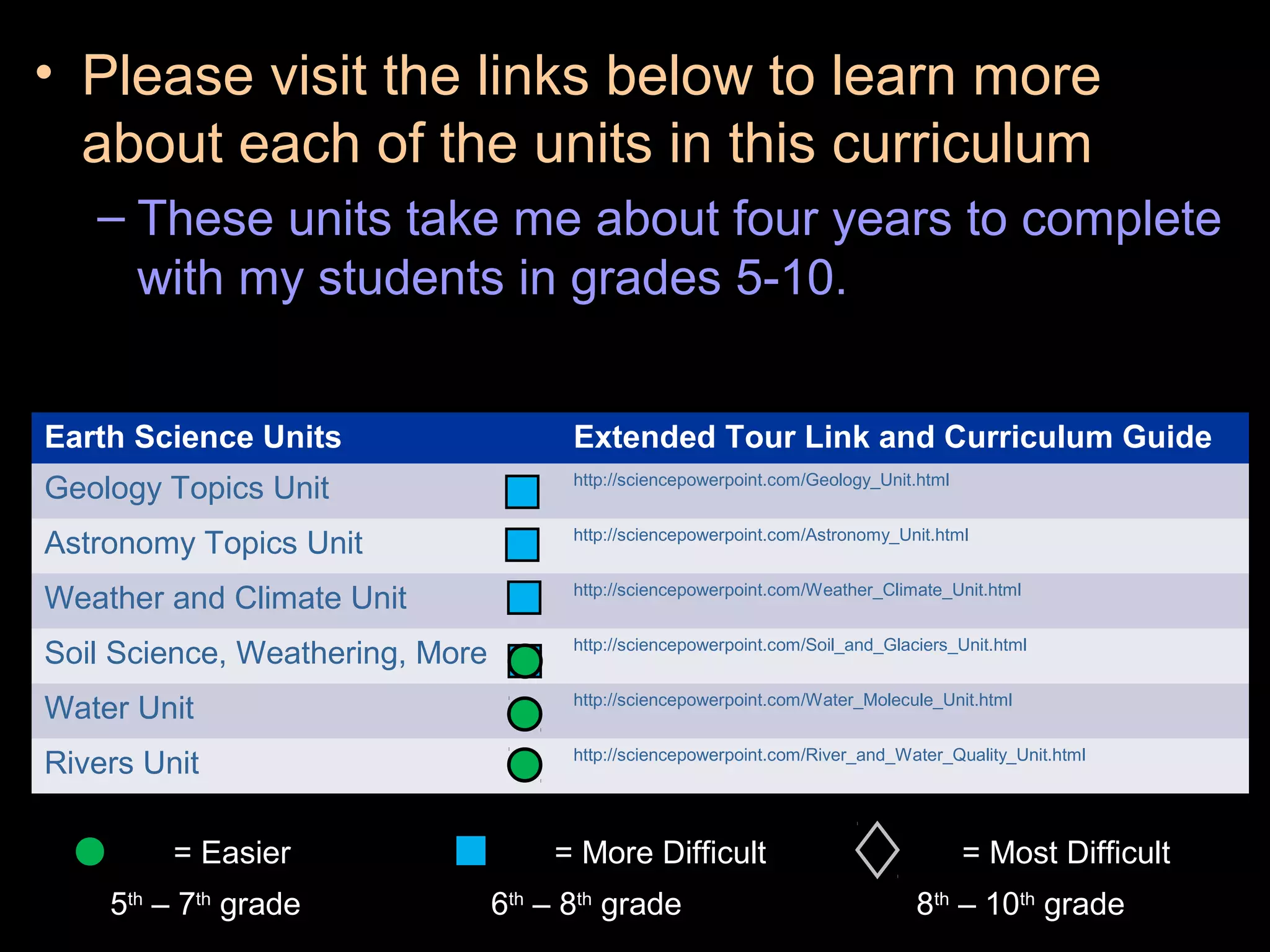
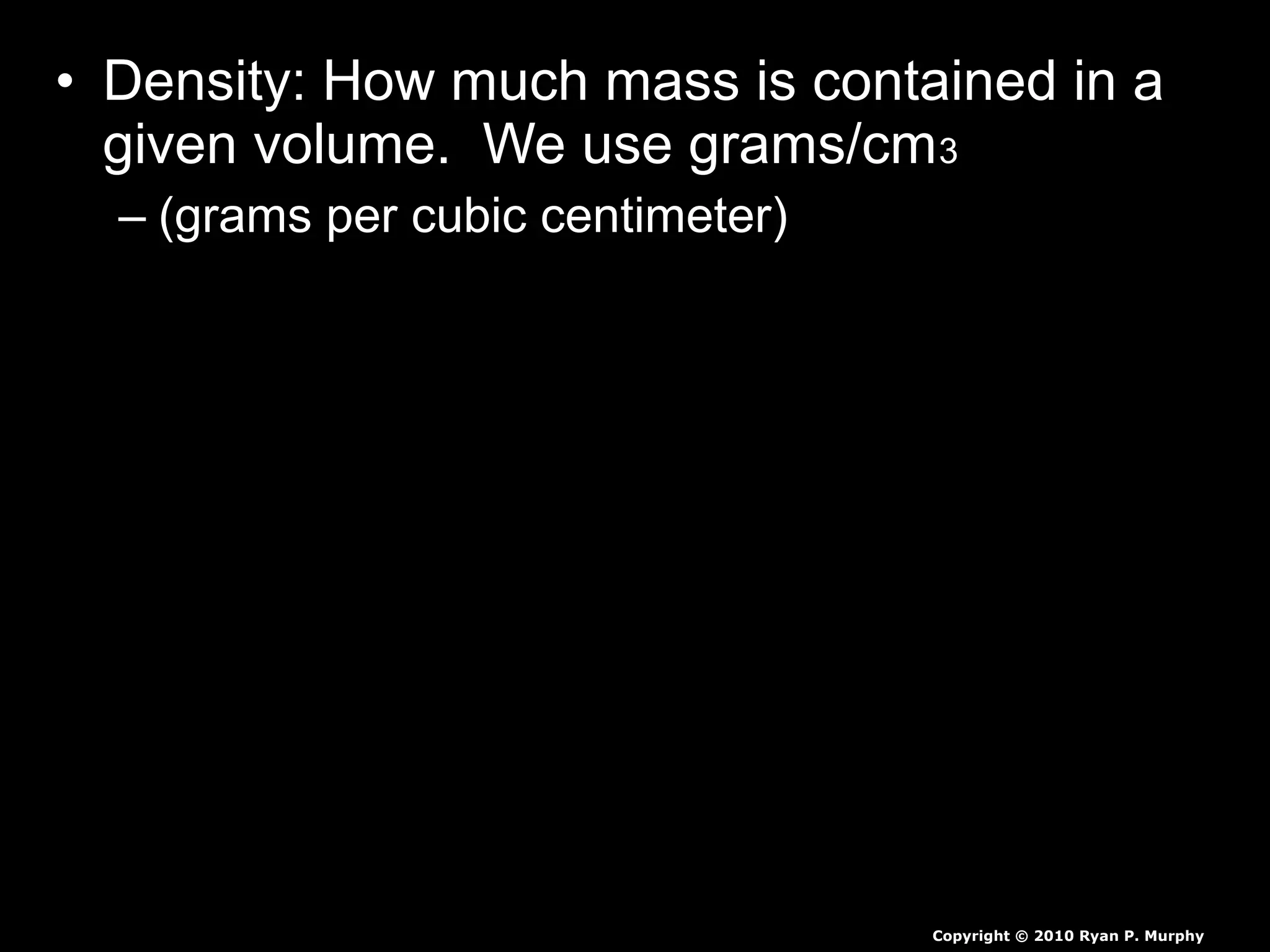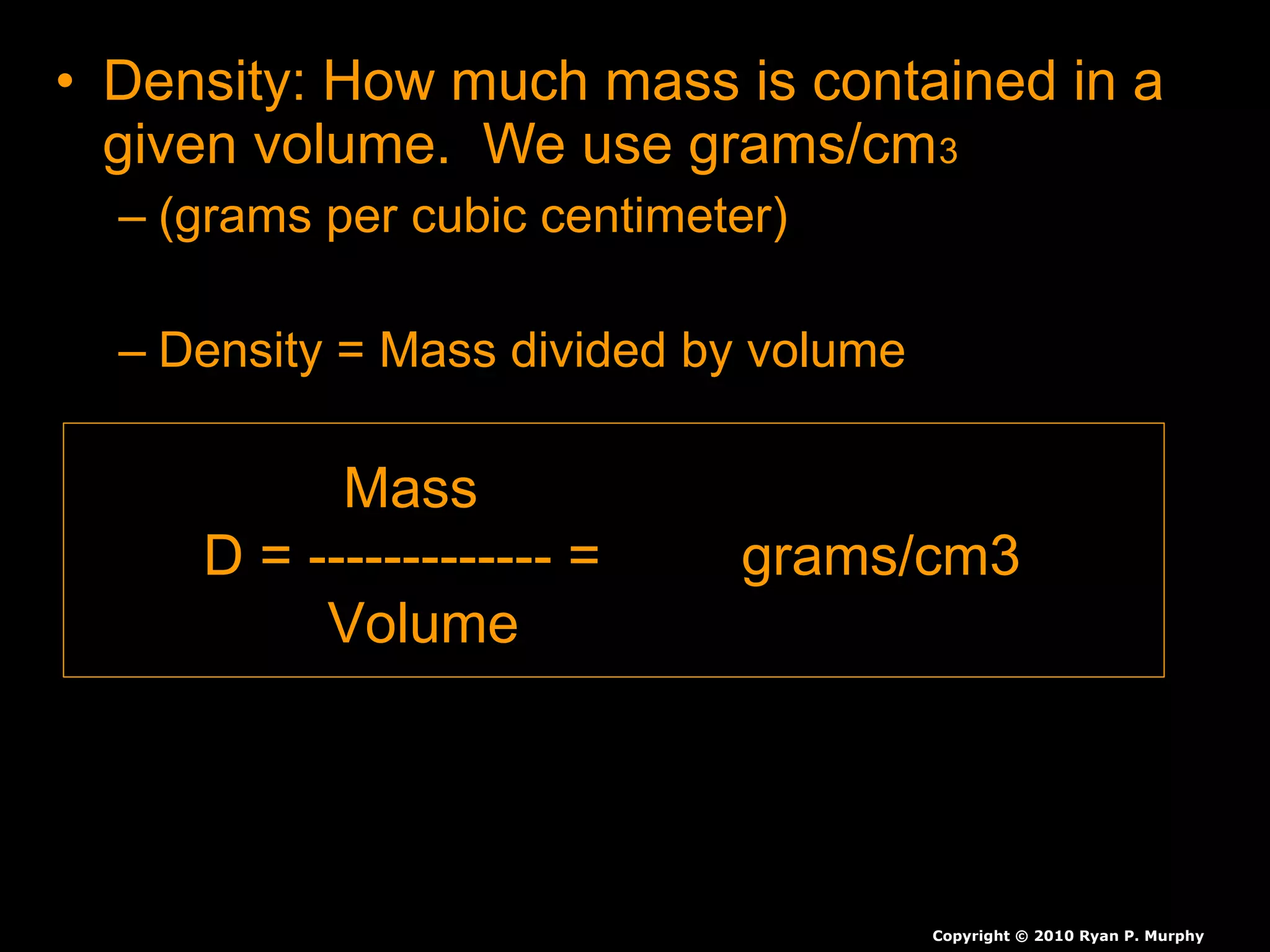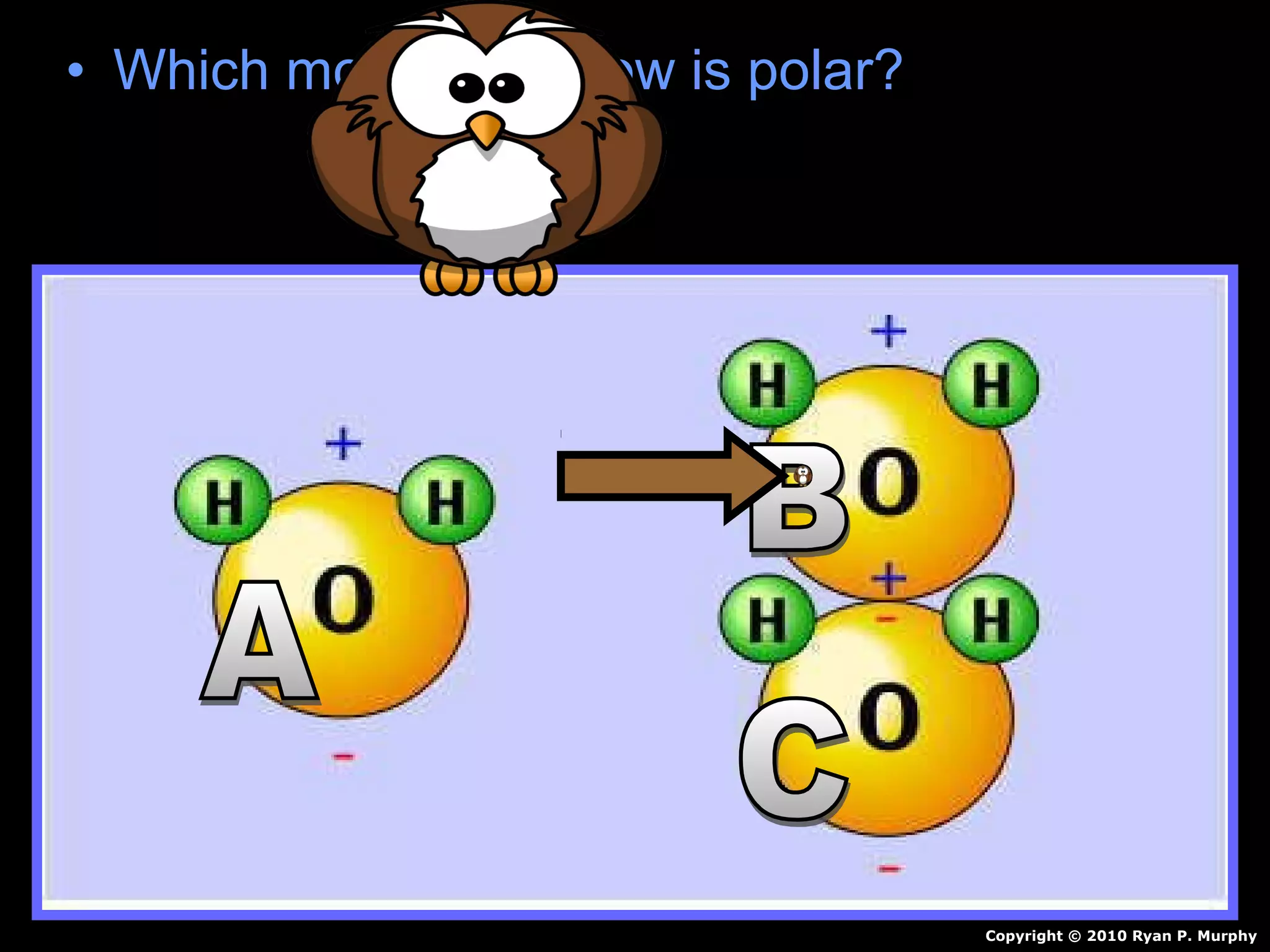The document provides instructions and information regarding a science class focused on the properties of water and related experiments. Key activities include building lava lamps, exploring the effects of detergent on milk, and understanding basic concepts of volume and density. It emphasizes the importance of taking clear notes and completing assigned worksheets related to these topics.