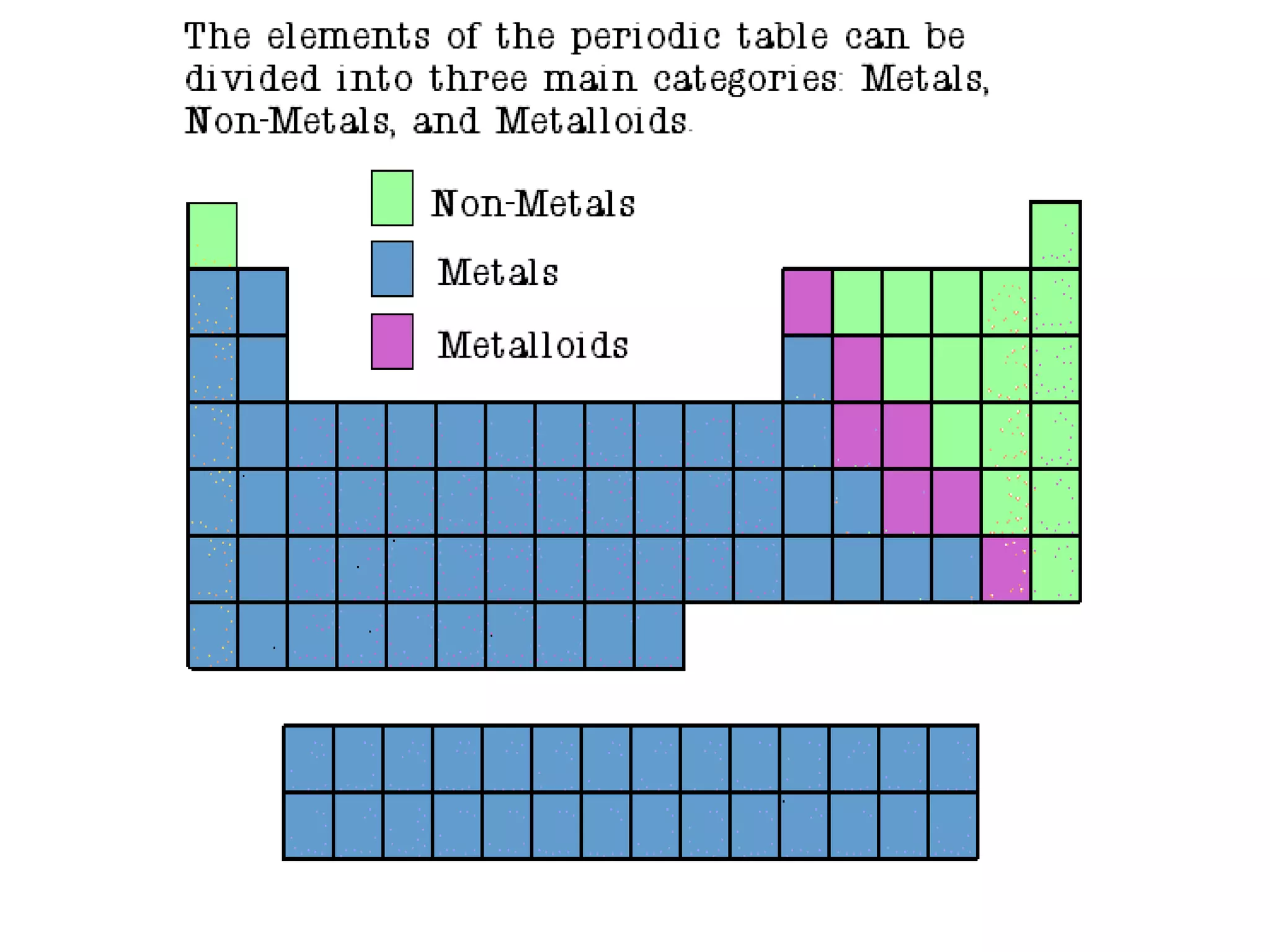
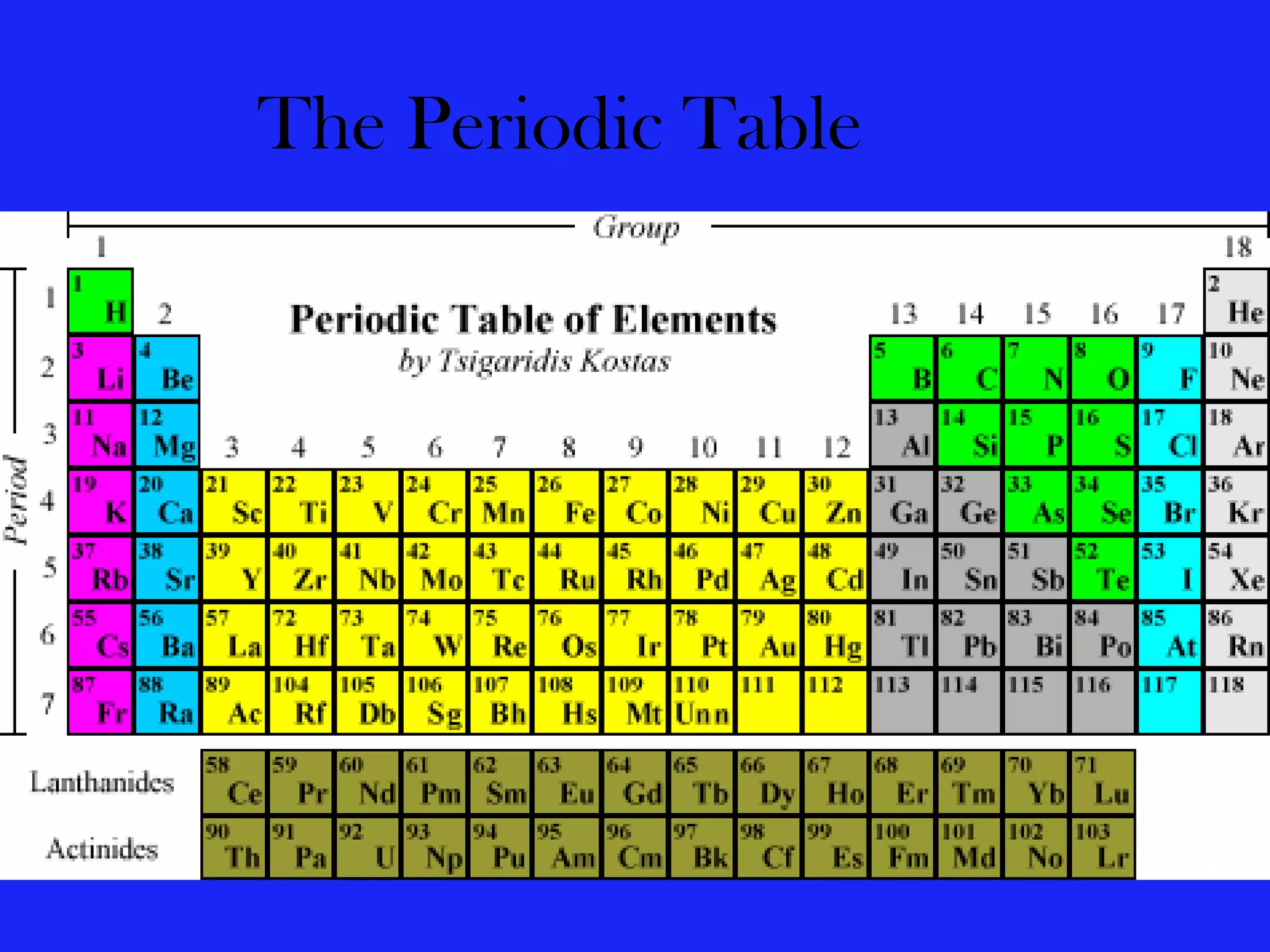
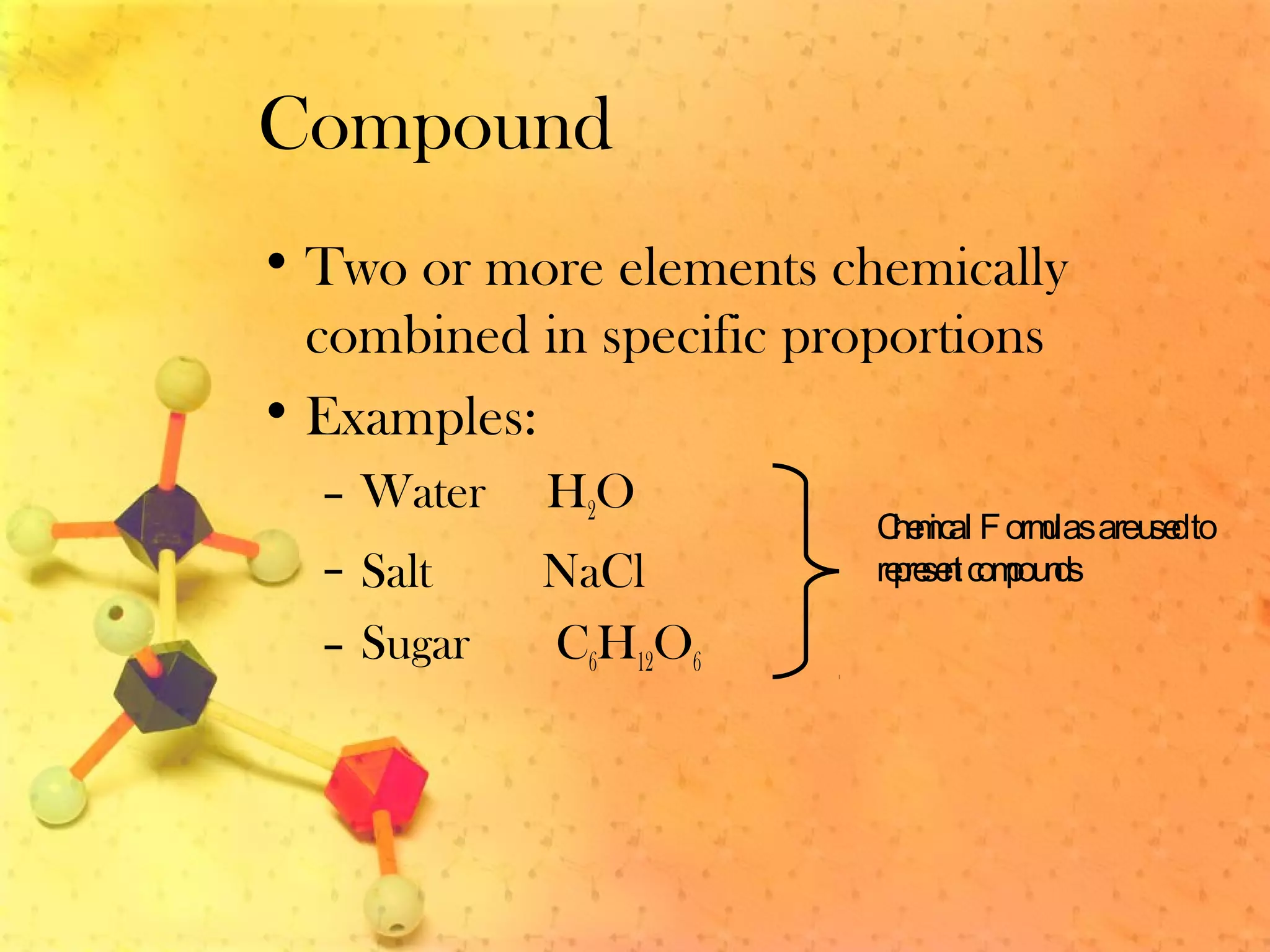
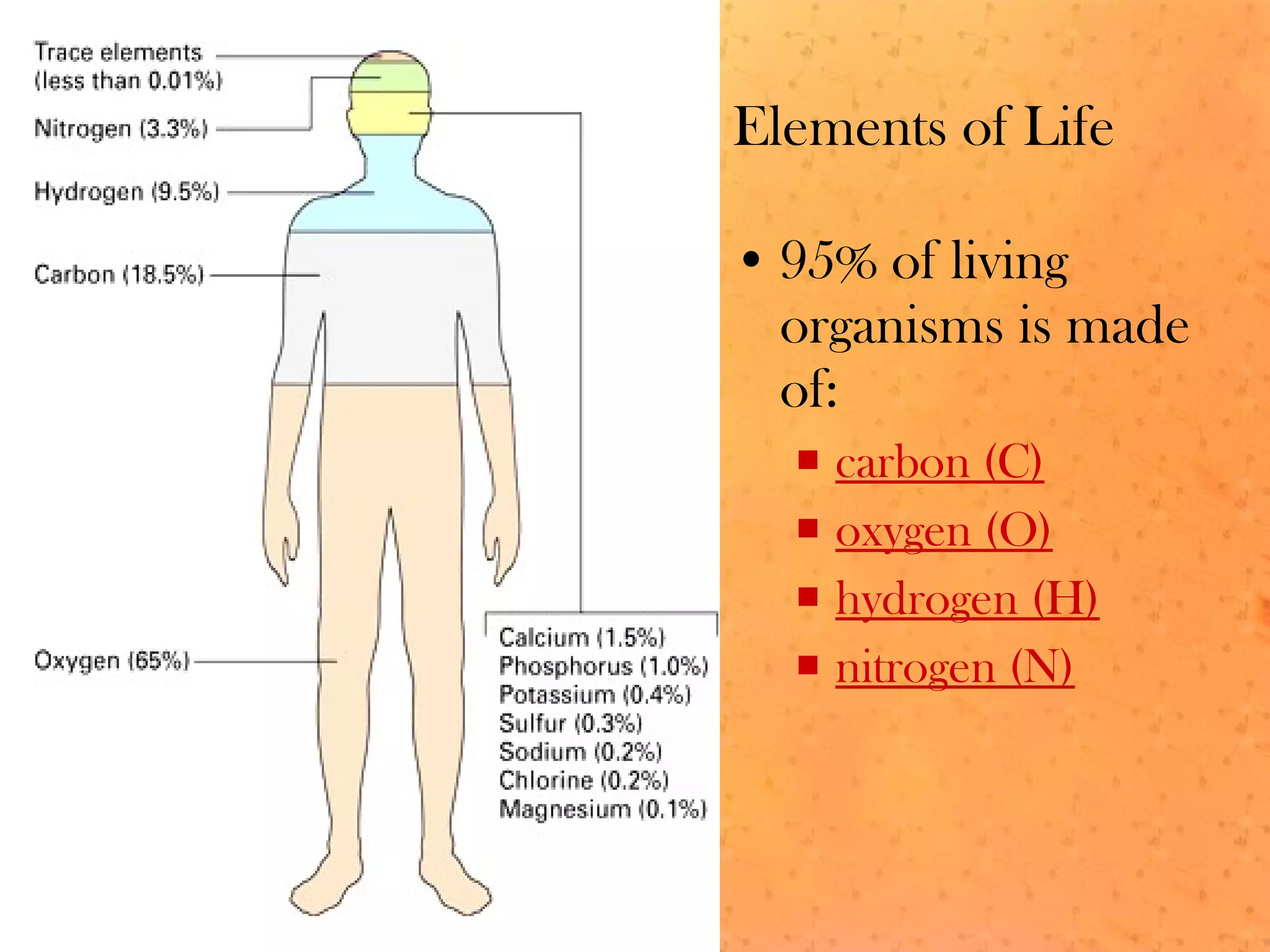
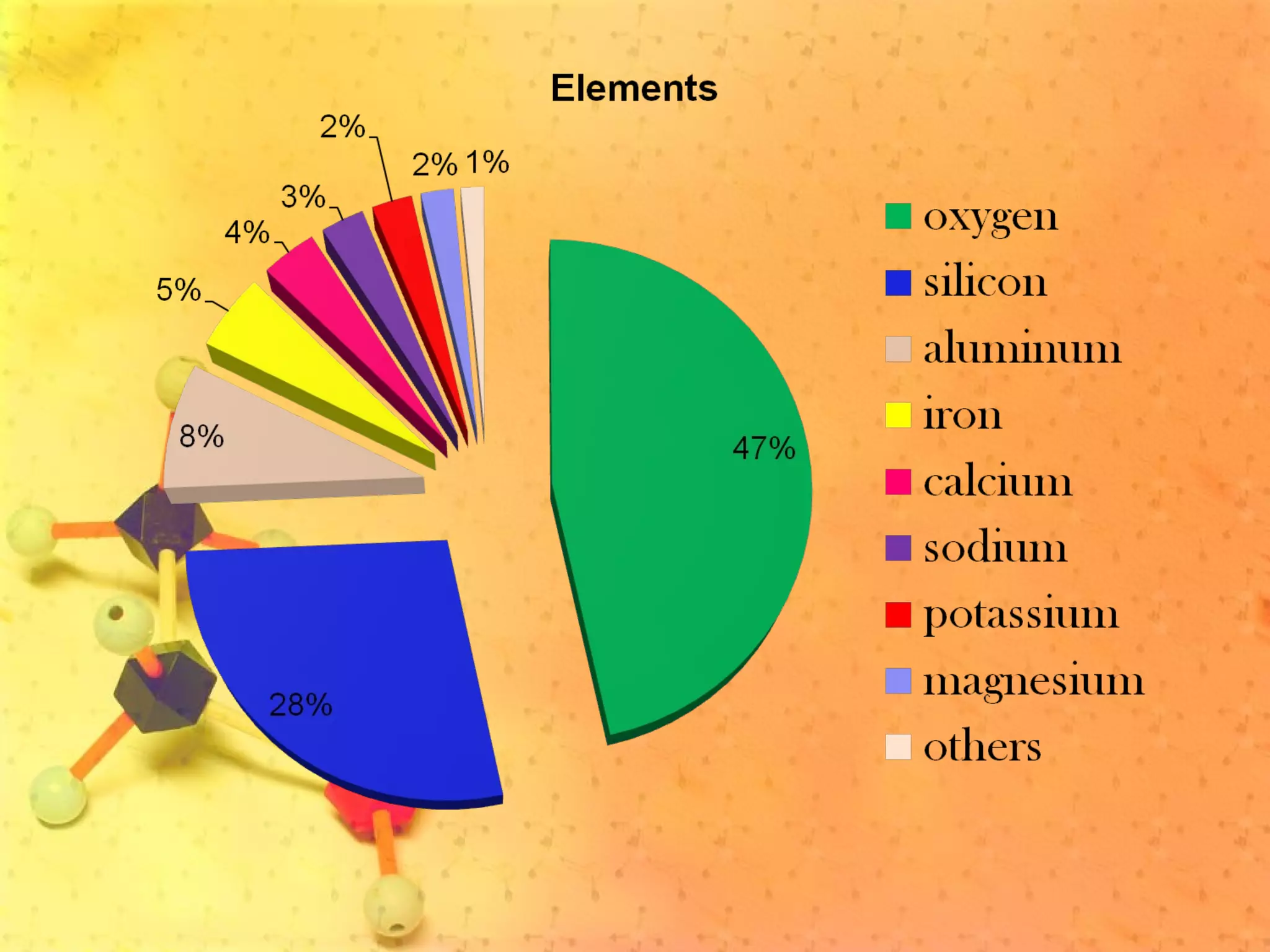
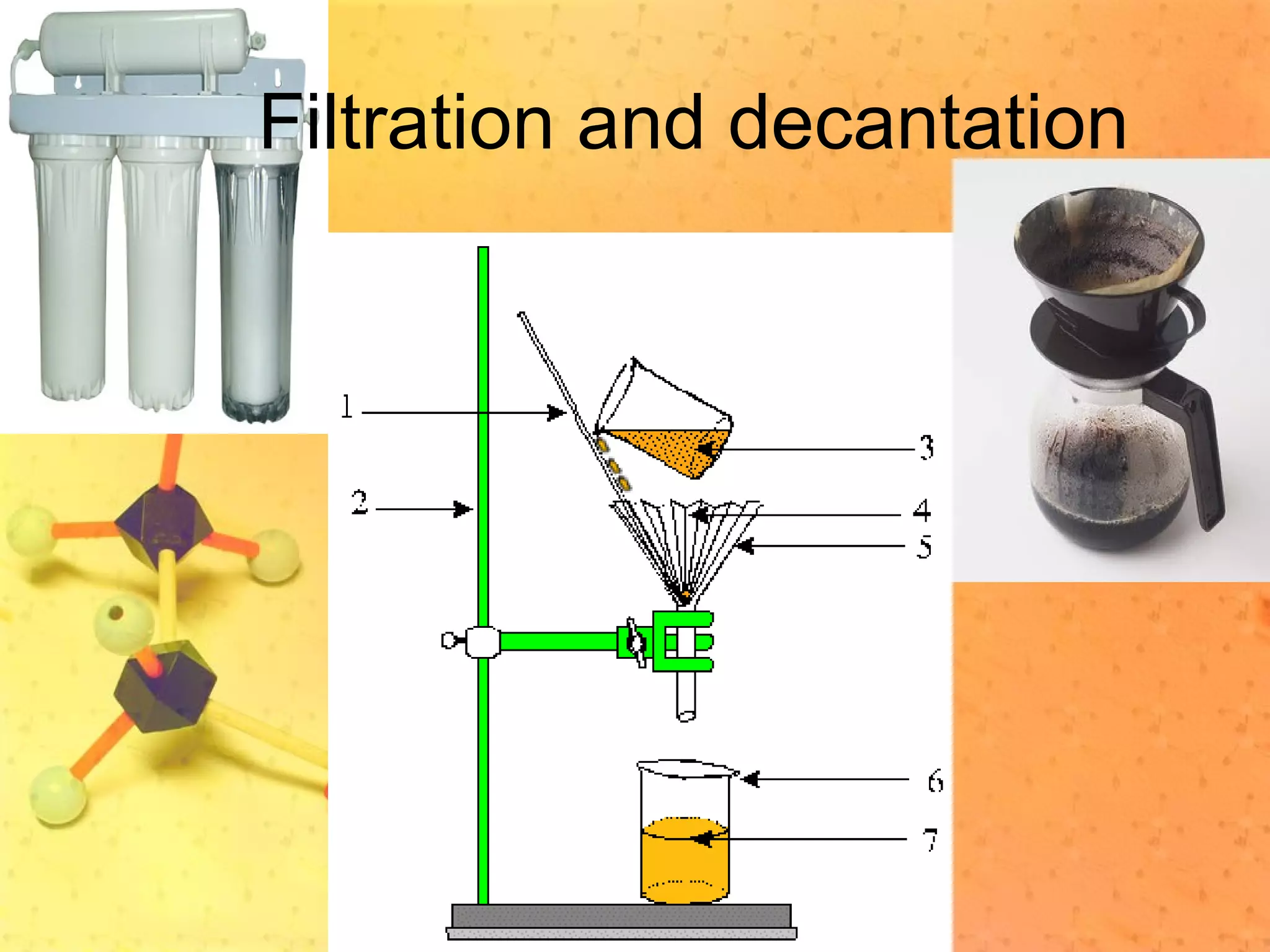
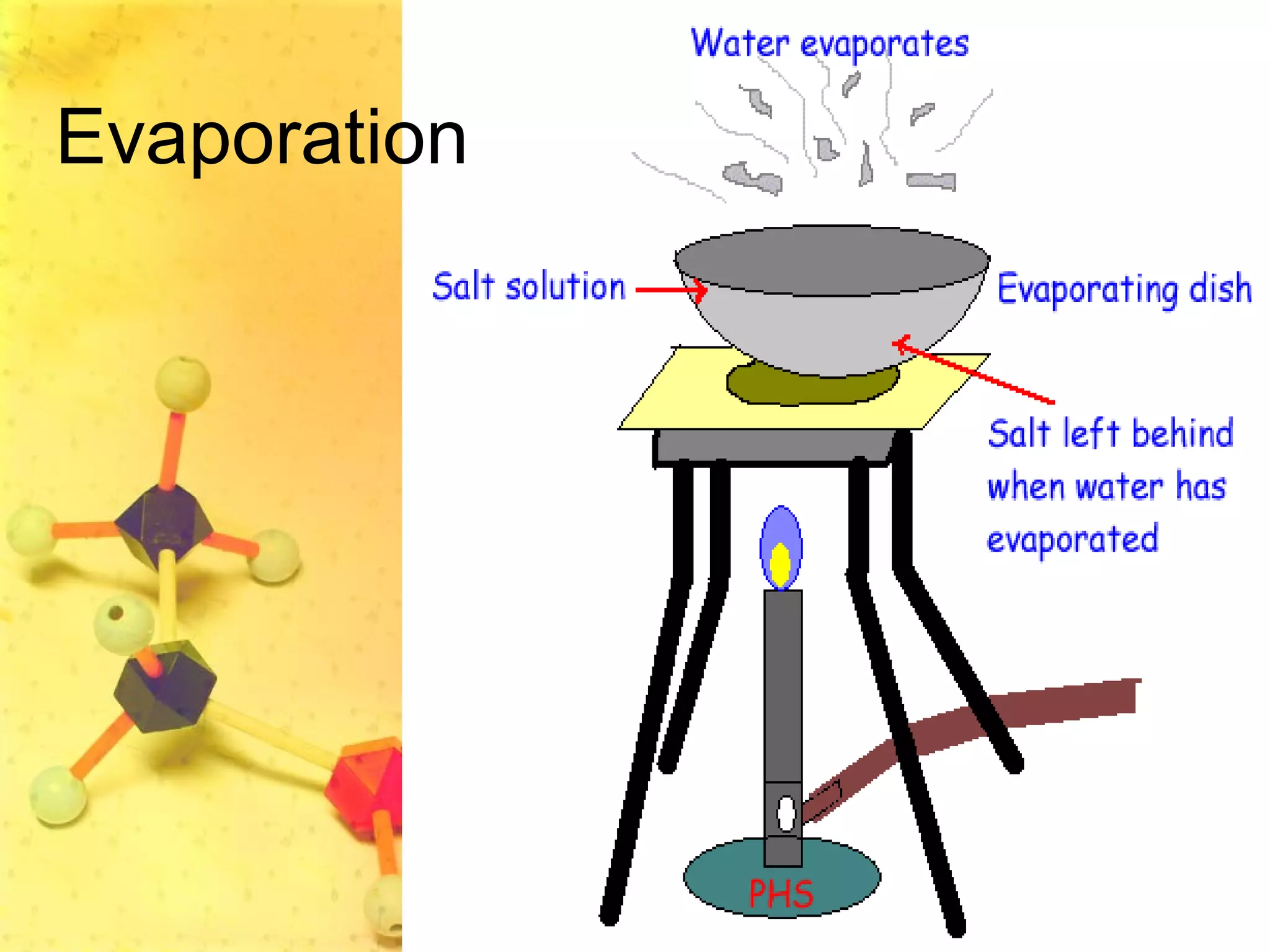
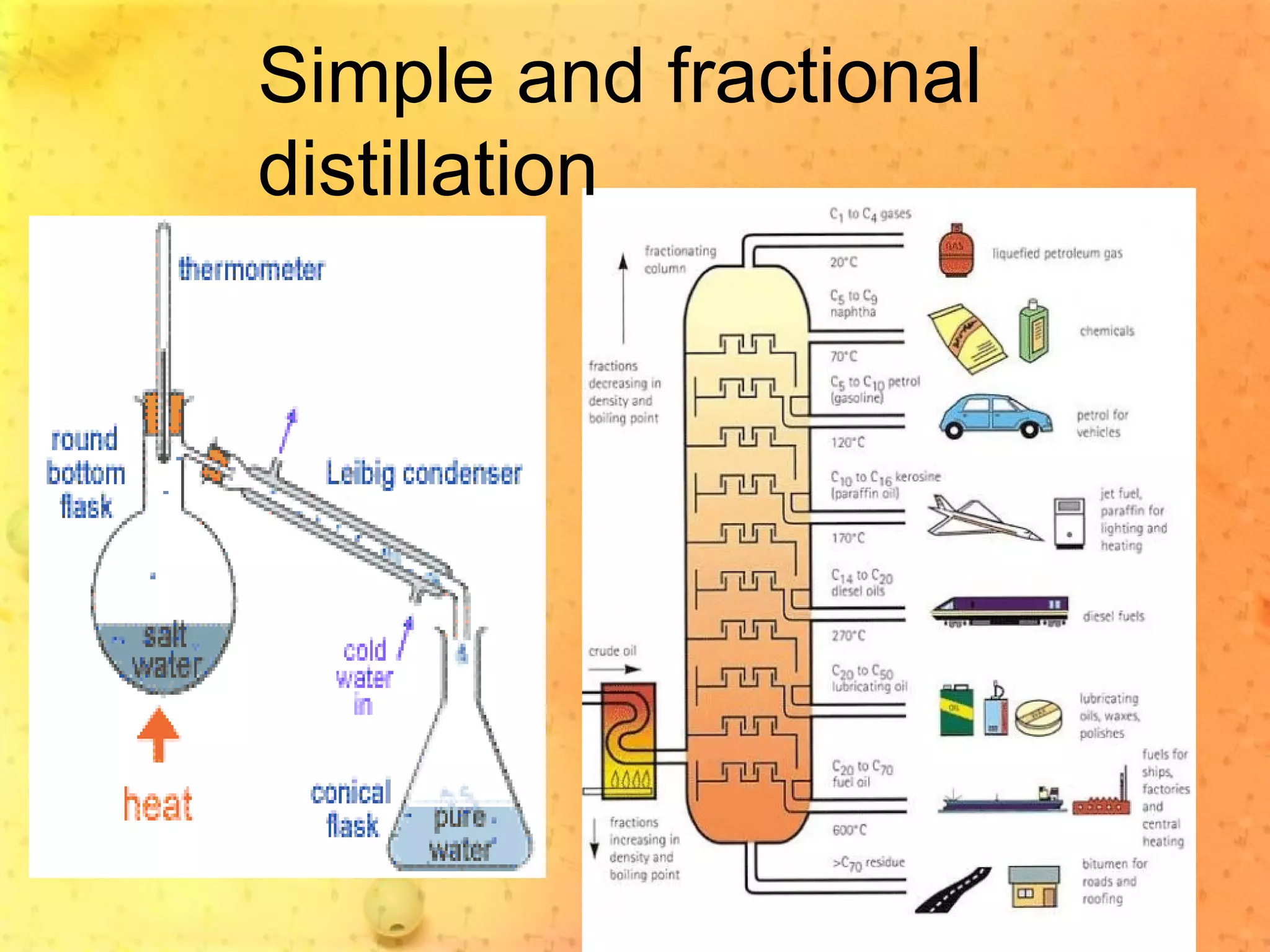
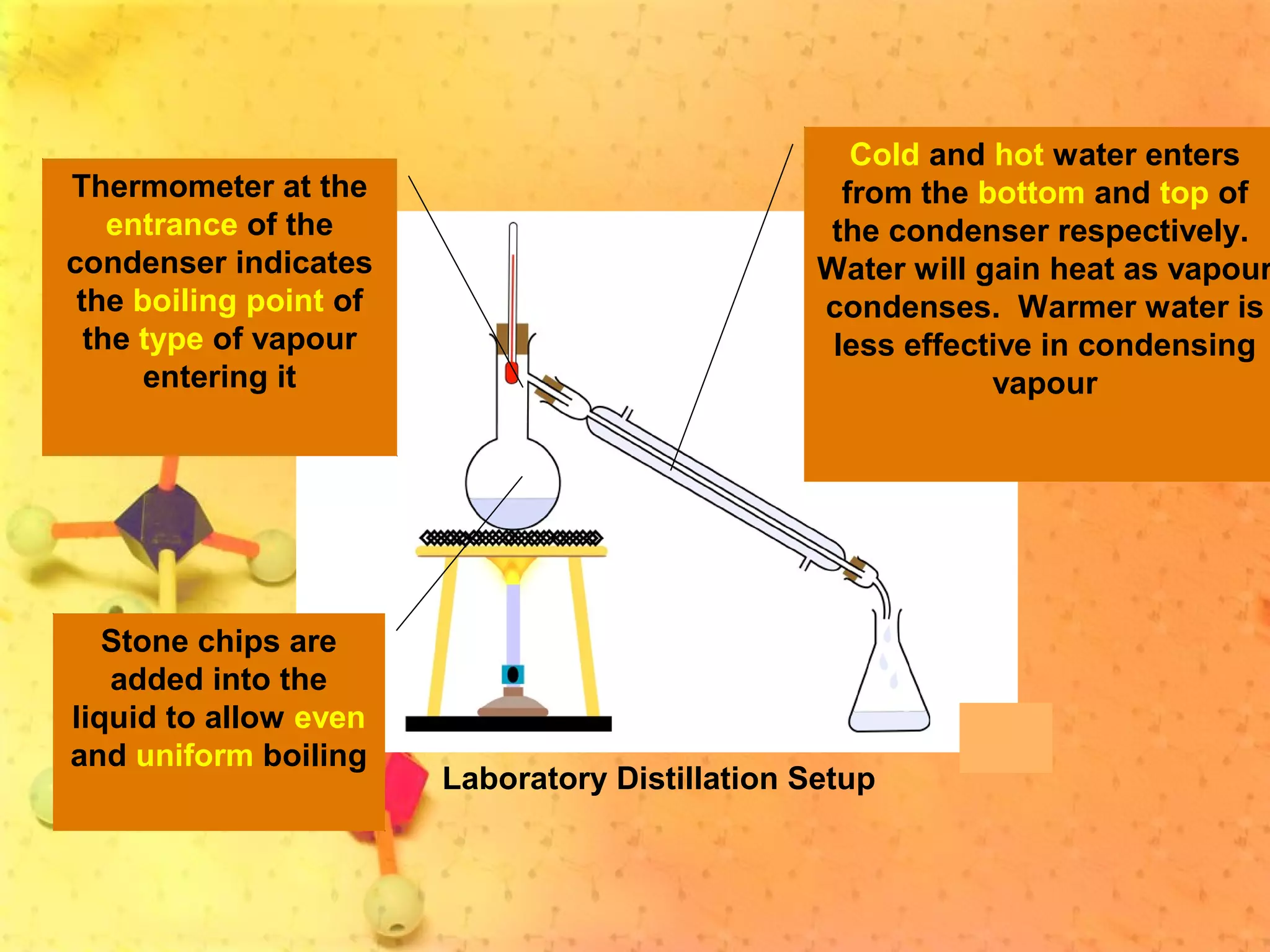
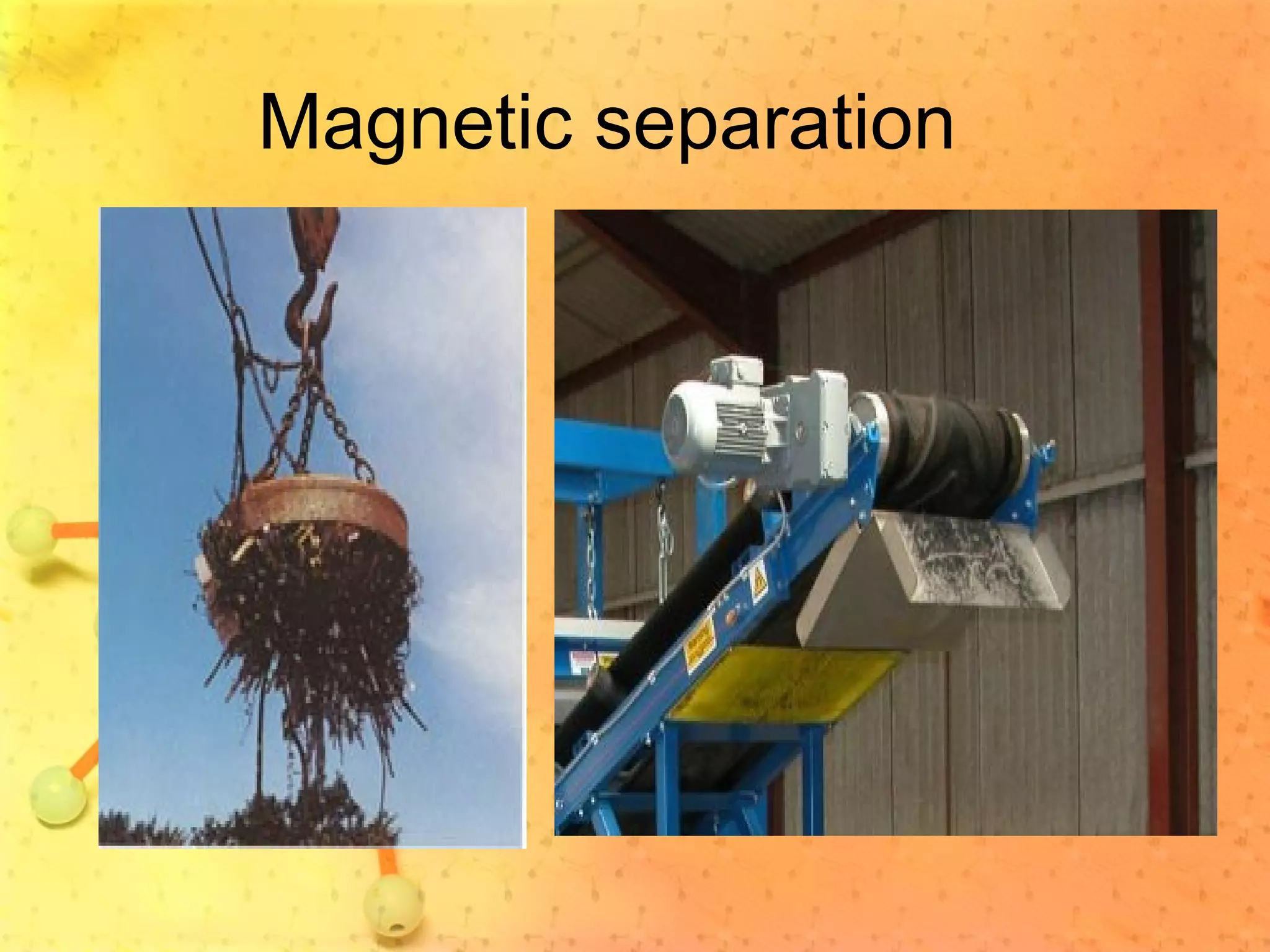
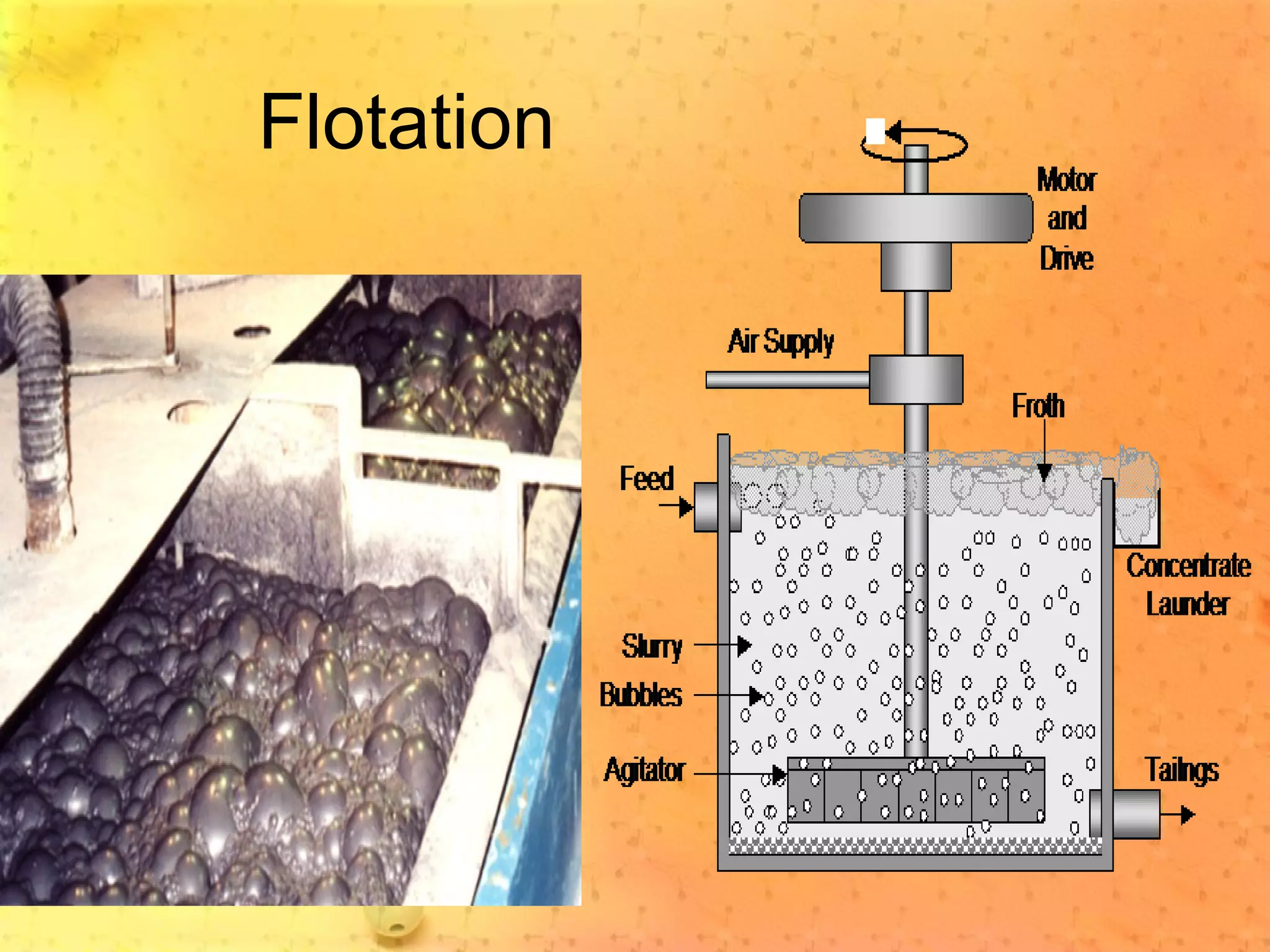
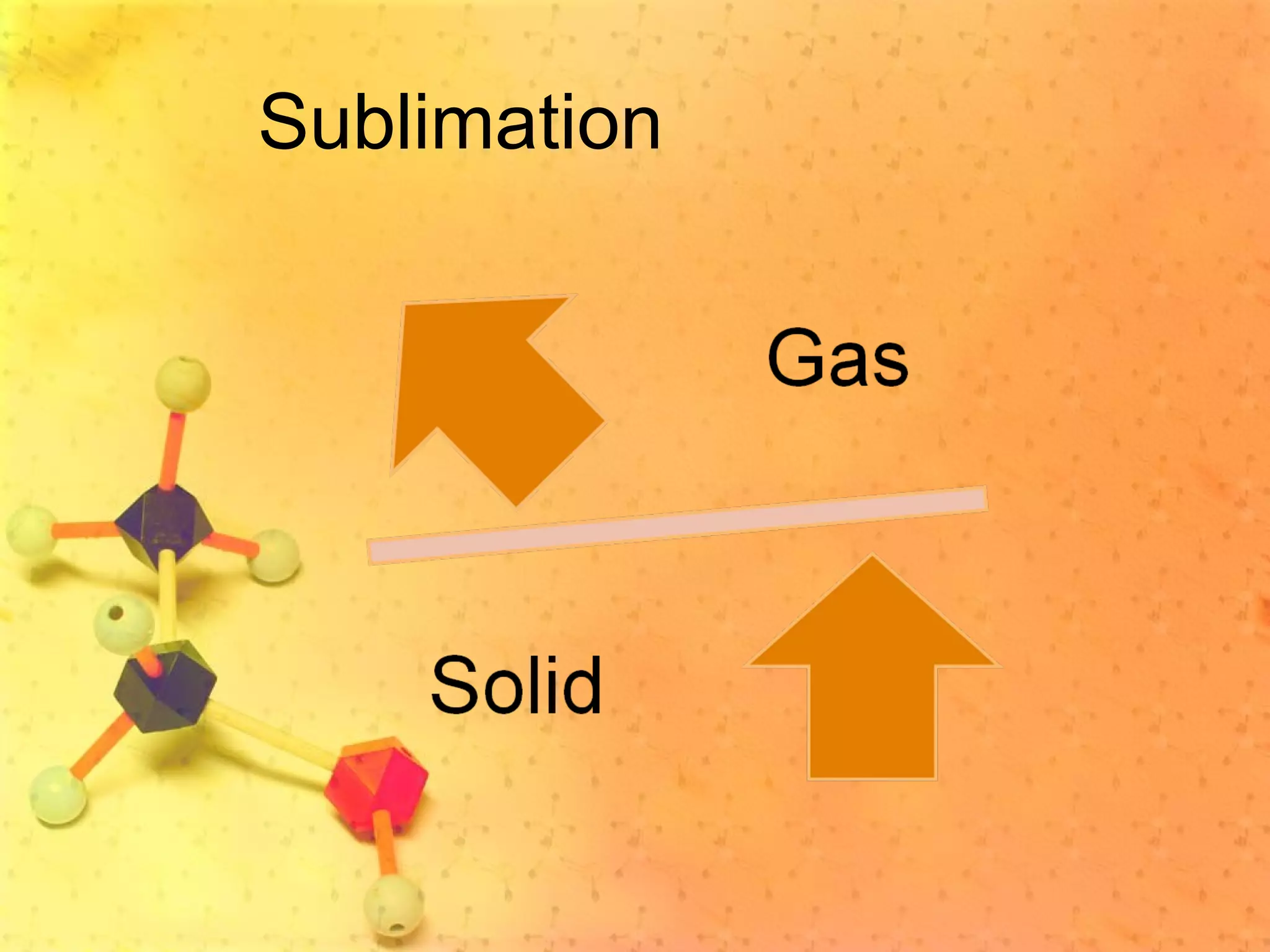
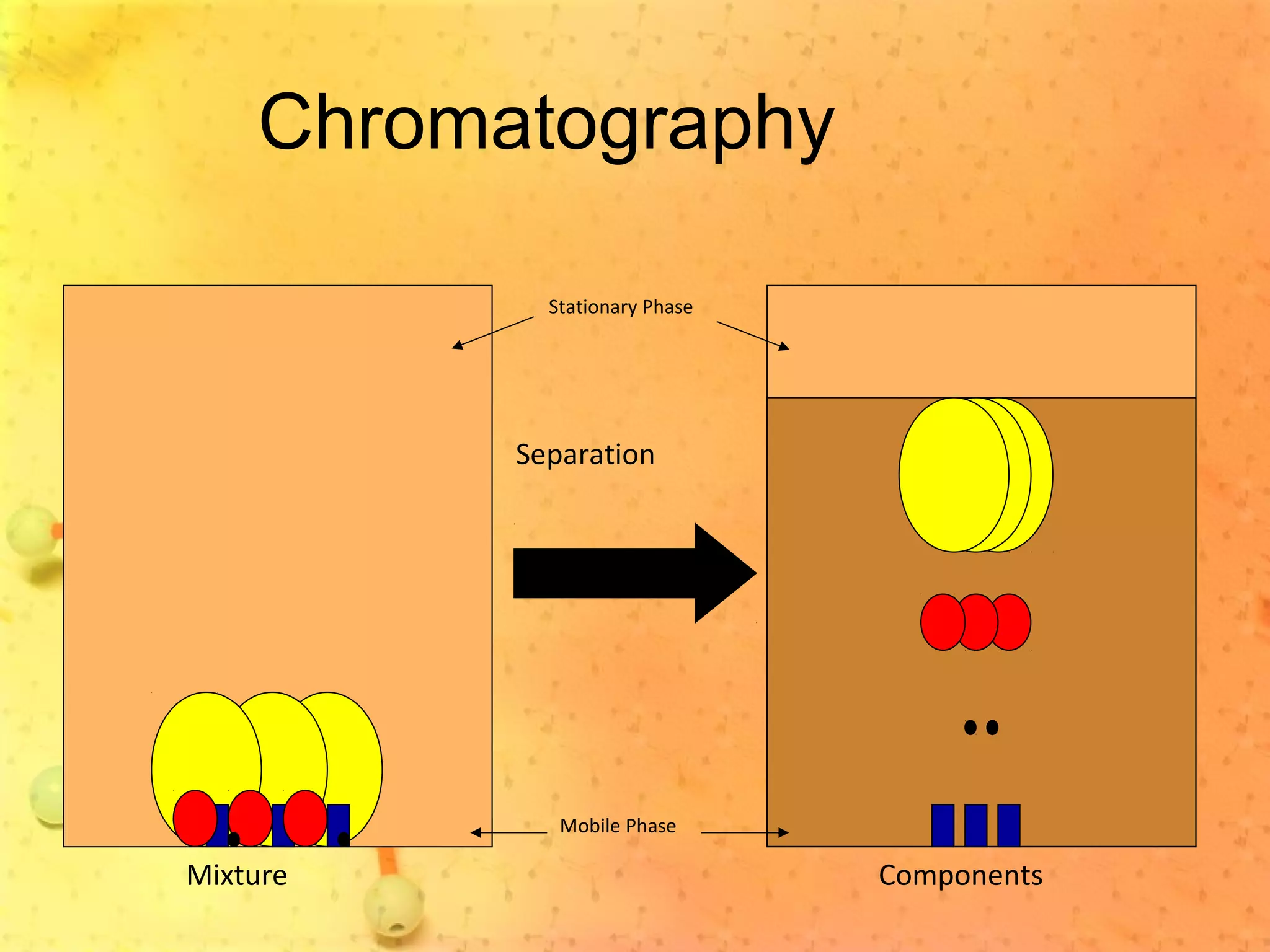
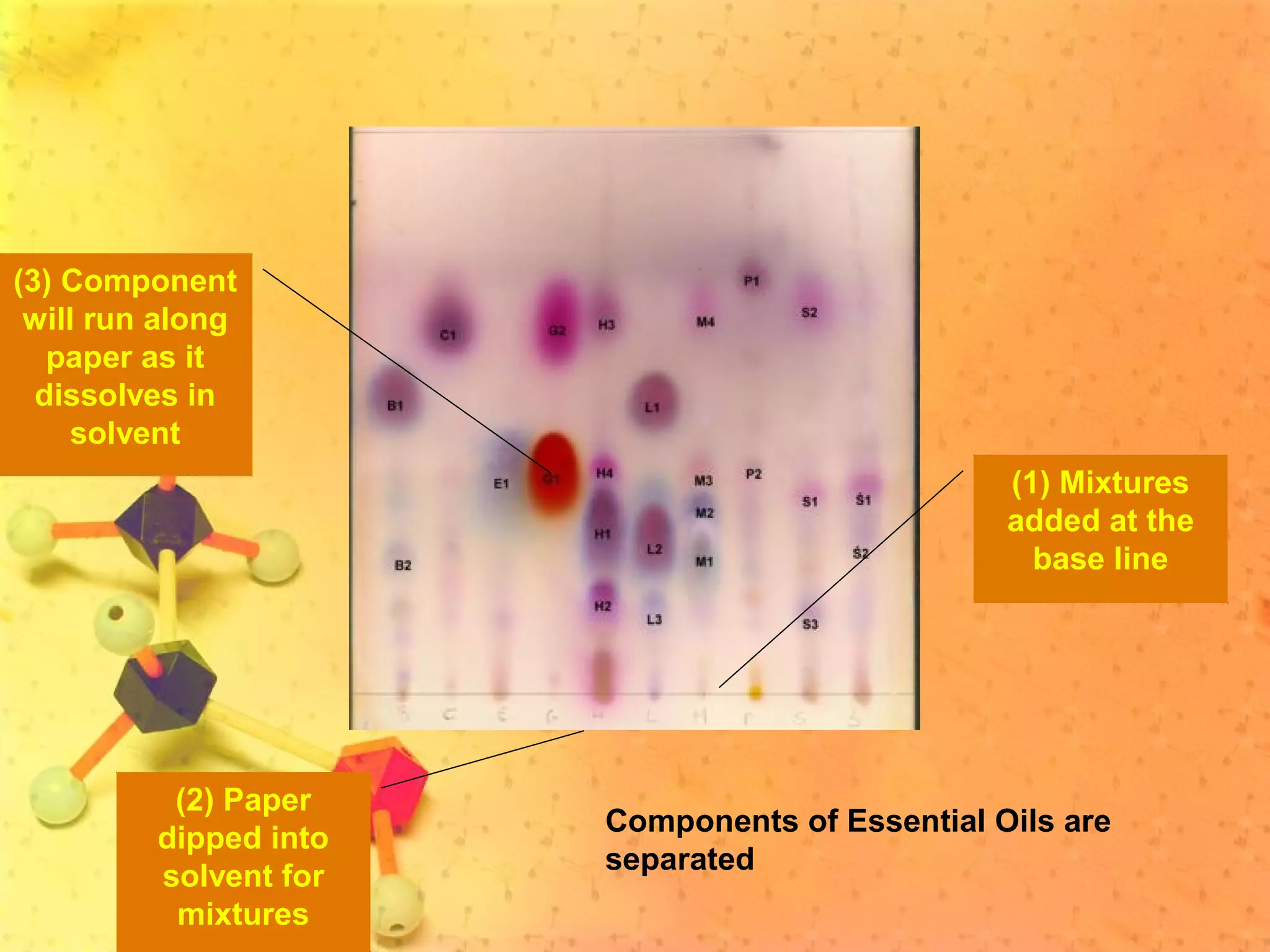
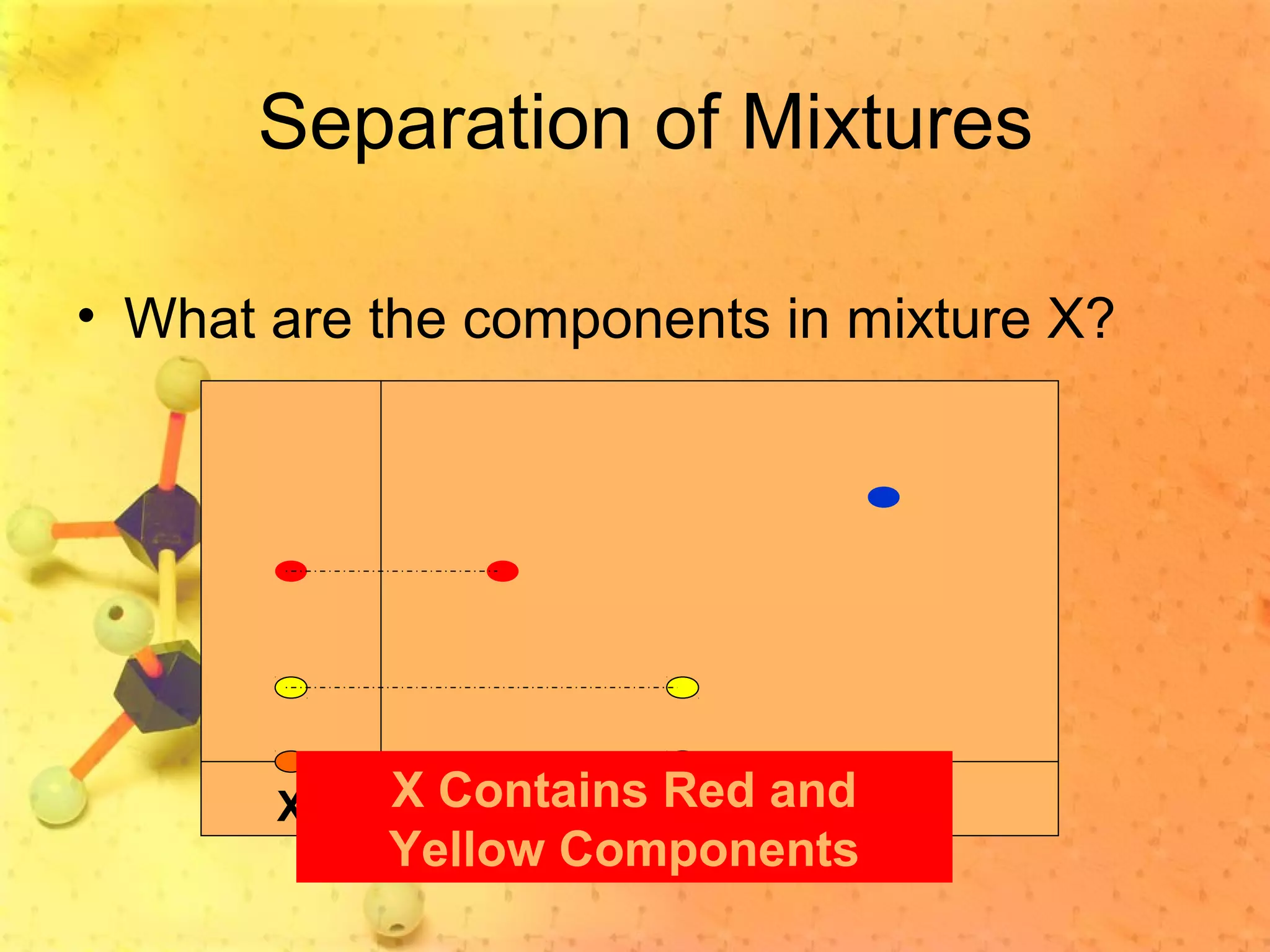
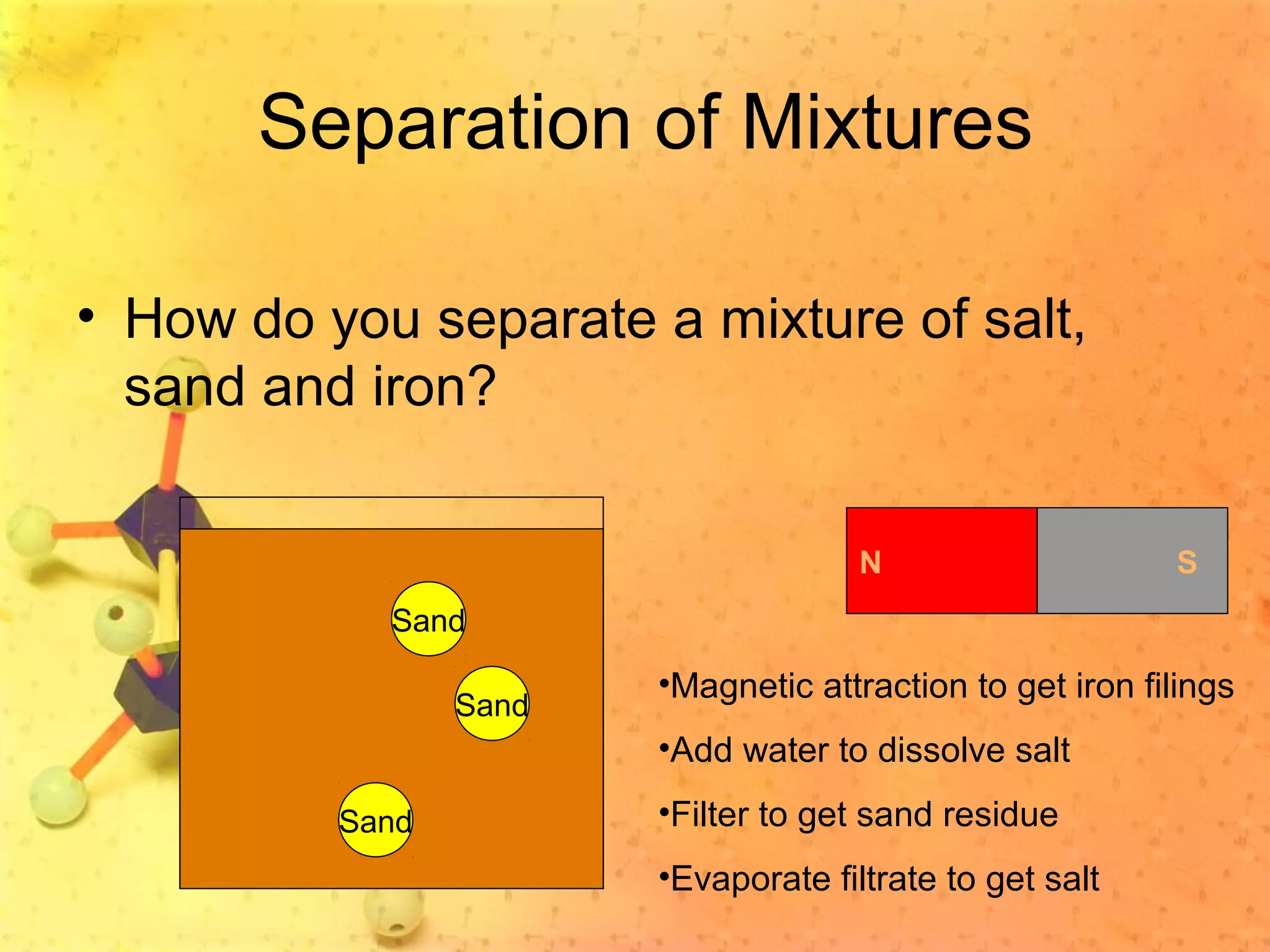
The document discusses various elements and their properties. It describes carbon as the most important element, which can form long chains and complex molecules, some of which are living matter. Gold is described as the most beautiful element, being metallic yet colorful and non-reactive. Mercury is identified as the most fascinating element as it is liquid at room temperature, though toxic. Iron is labeled the most useful element due to its versatility when alloyed. The document also notes that hydrogen is the most abundant element in the universe.