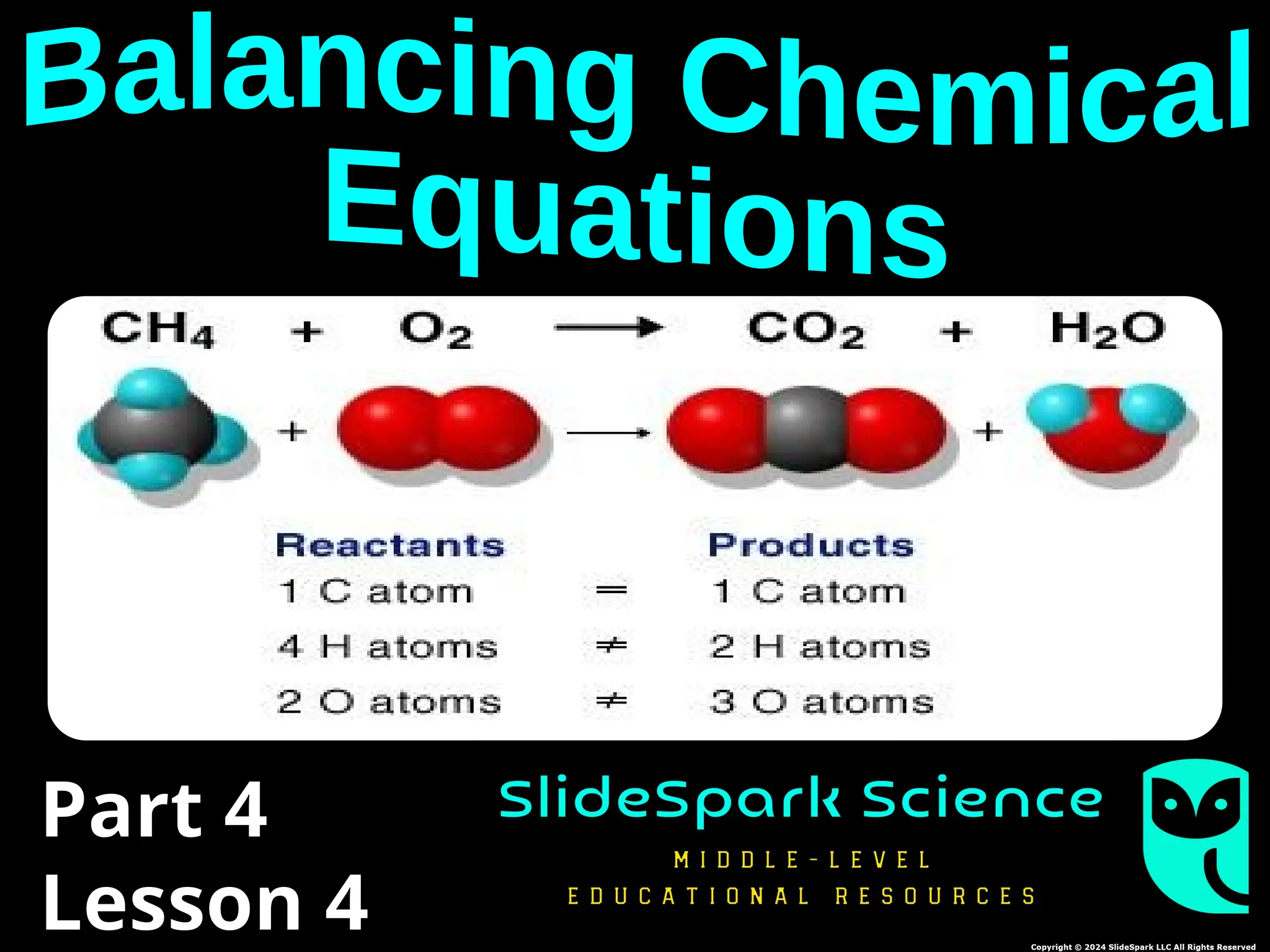
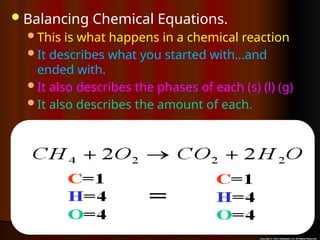
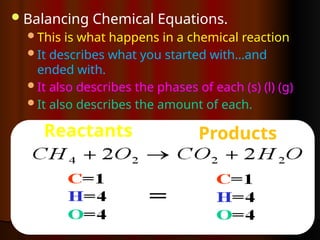
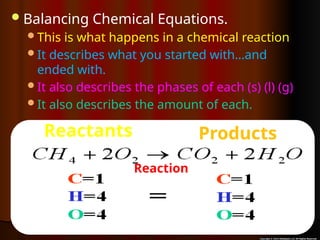
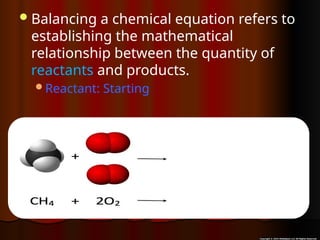
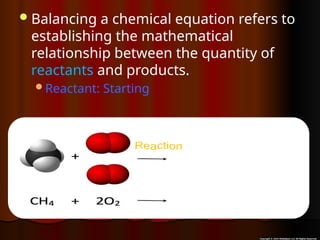
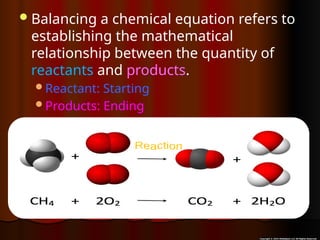
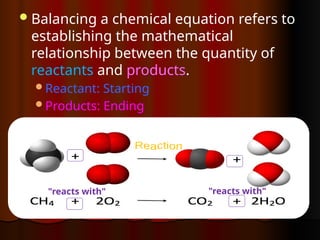
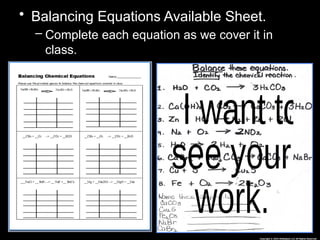
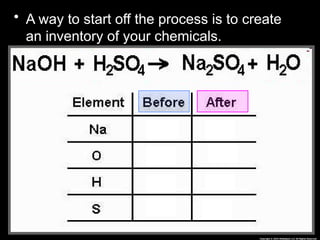
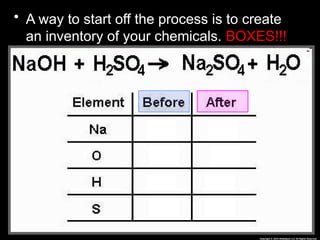
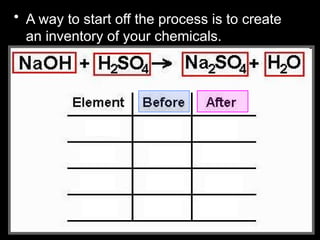
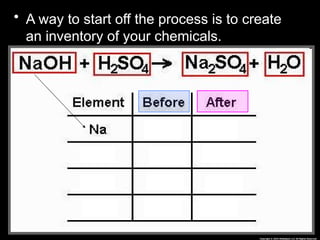
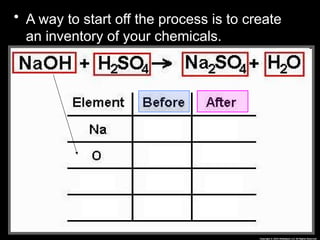
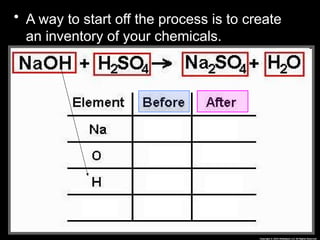
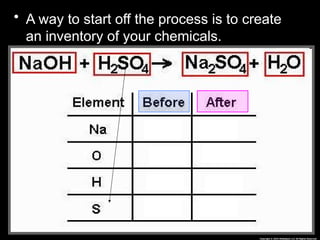
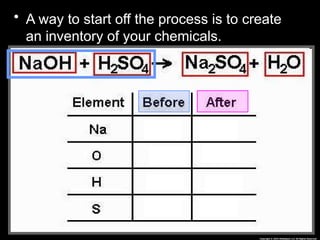
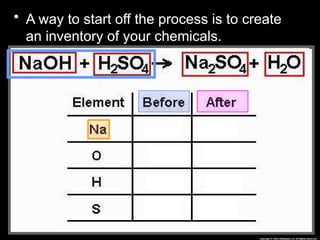
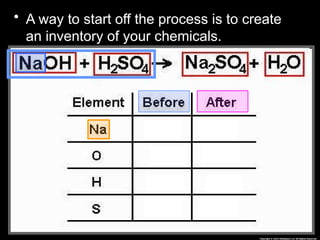
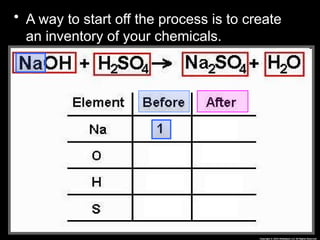
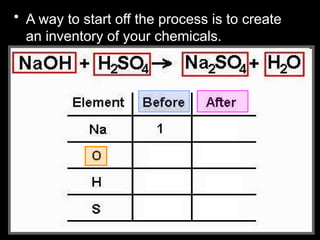
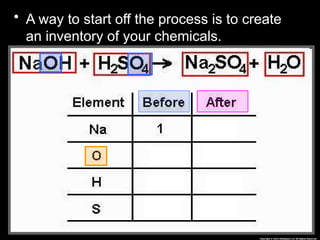
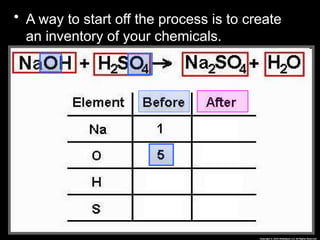
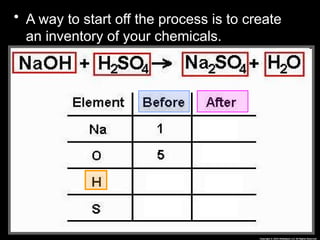
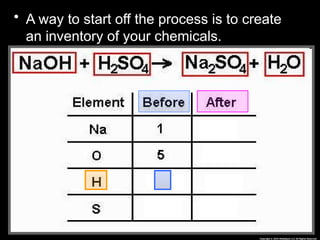
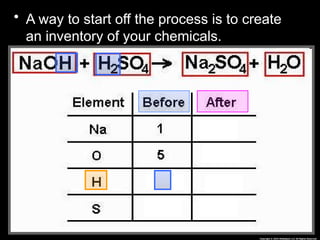
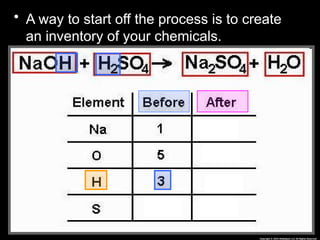
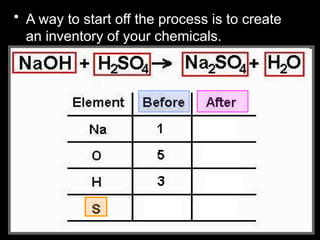
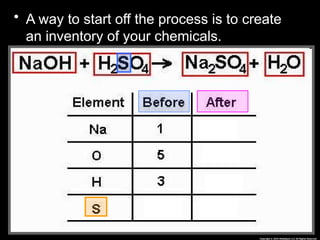
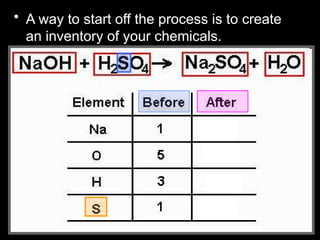
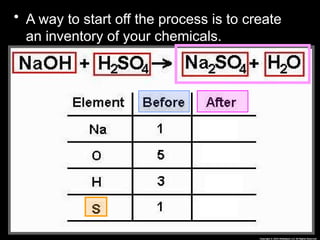
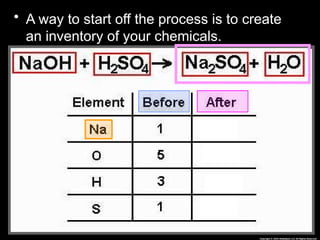
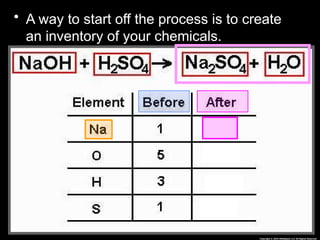
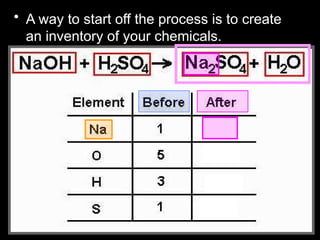
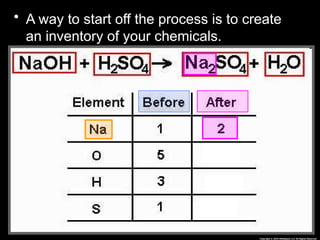
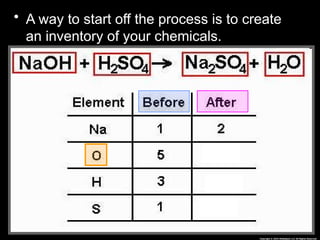
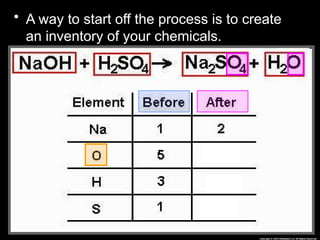
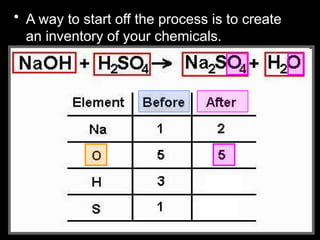
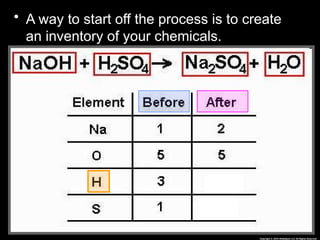
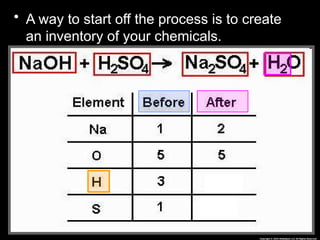
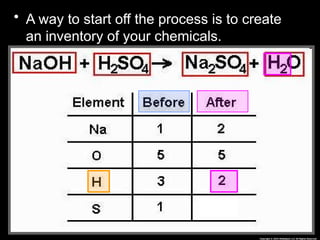
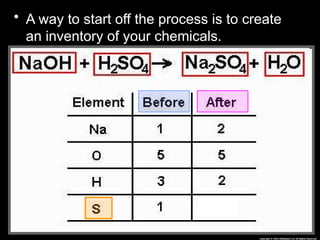
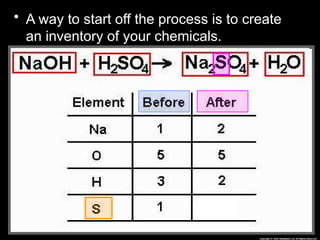
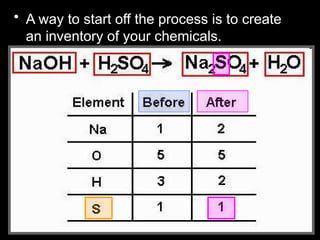
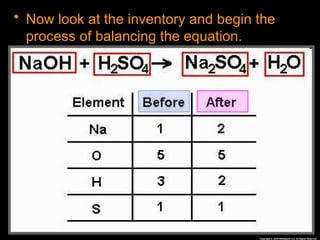
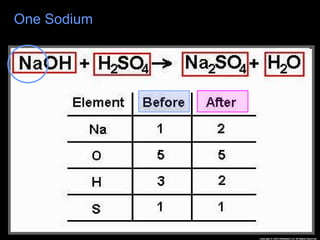
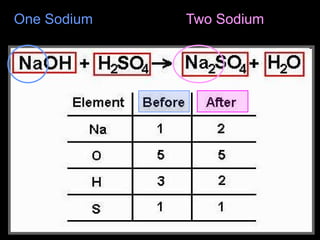
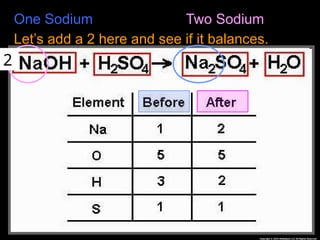
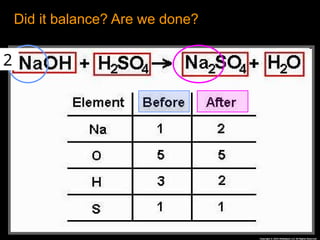
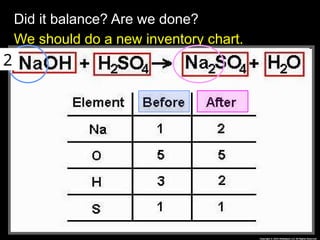
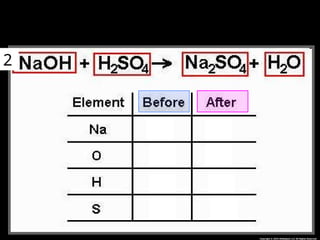
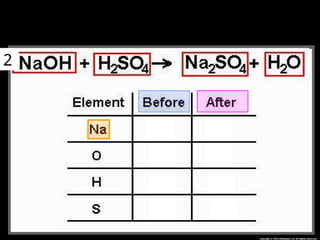
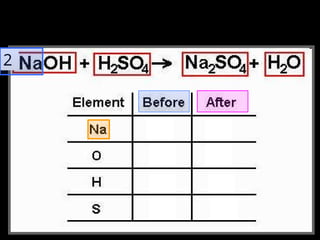
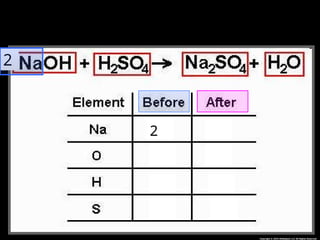
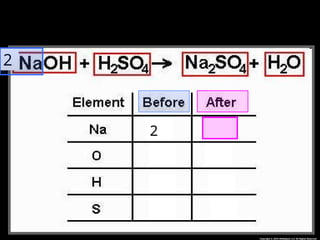
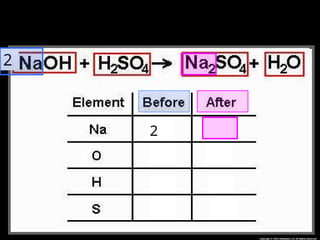
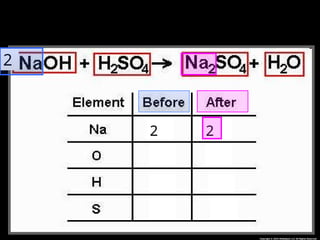
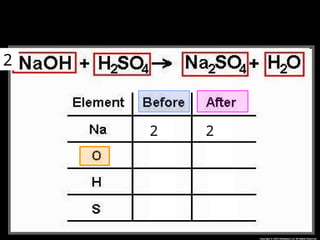
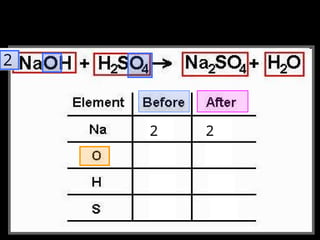
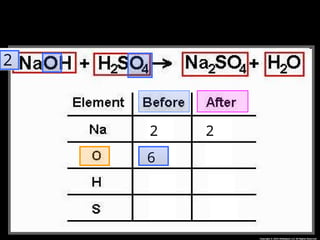
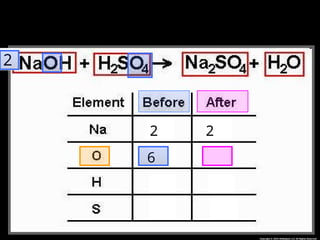
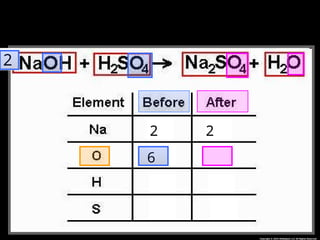
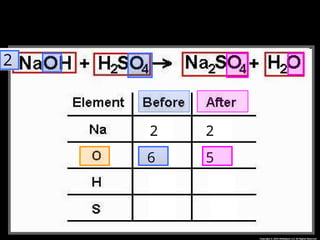
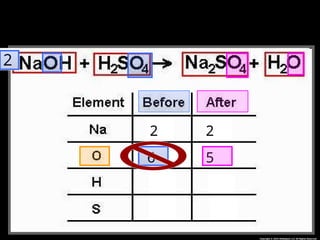
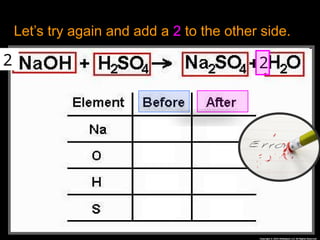
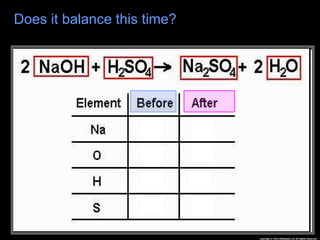
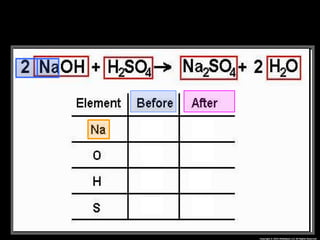
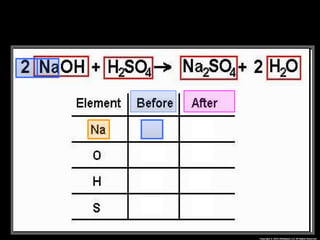
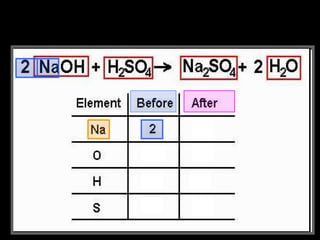
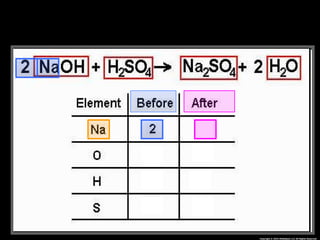
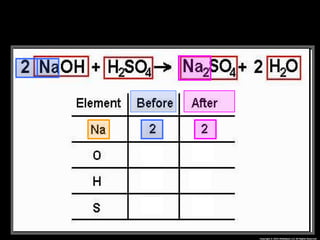
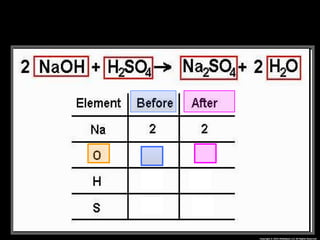
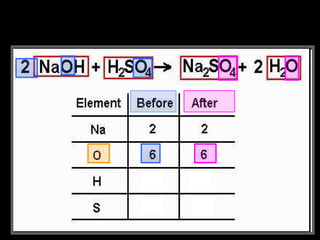
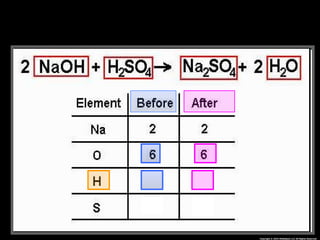
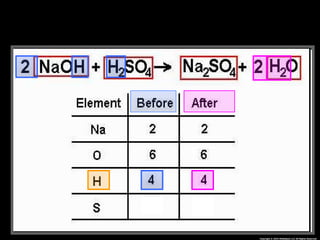
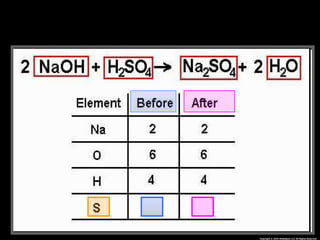
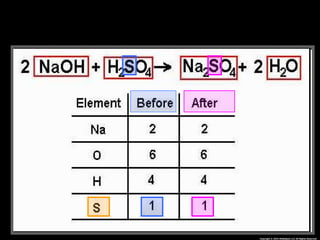
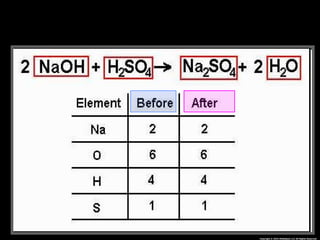
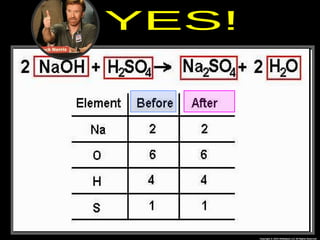
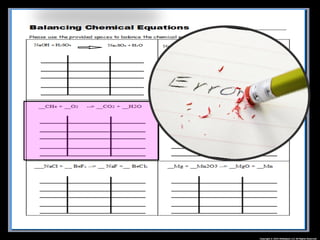
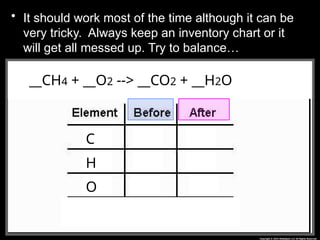
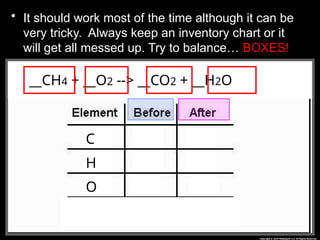
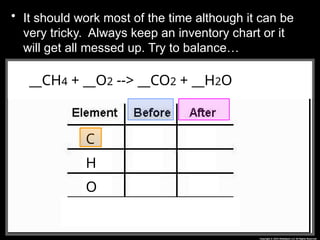
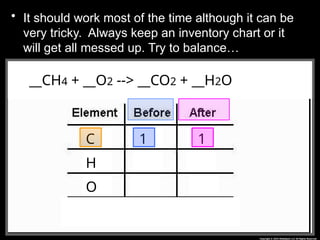
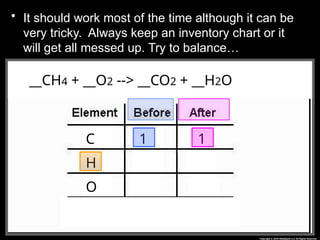
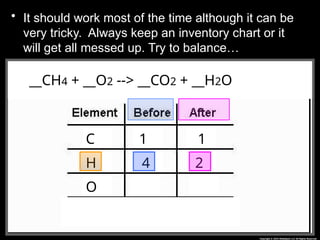
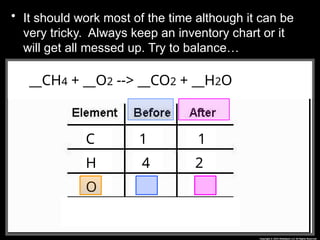
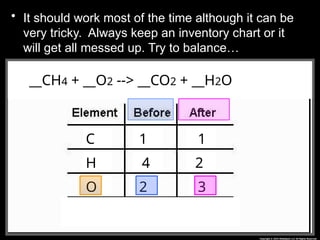
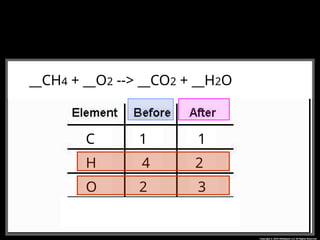
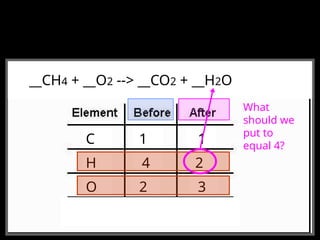
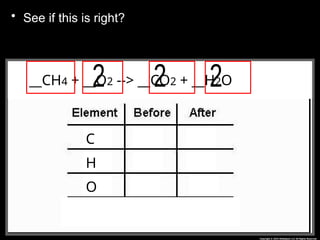
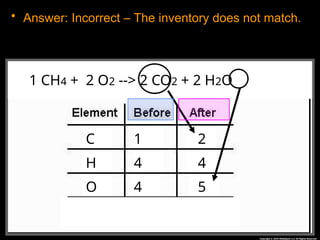
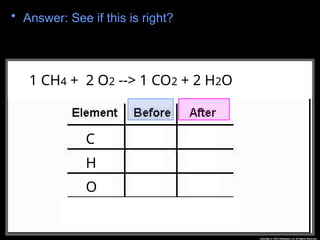
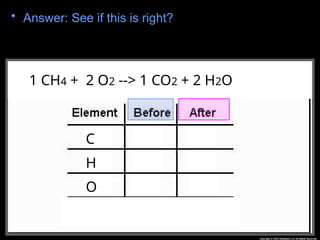
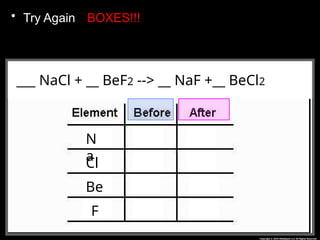
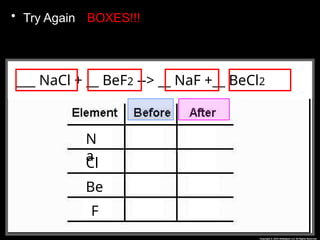
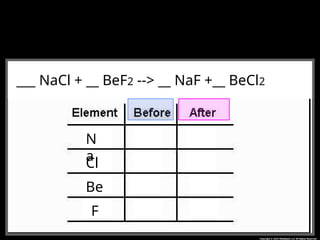
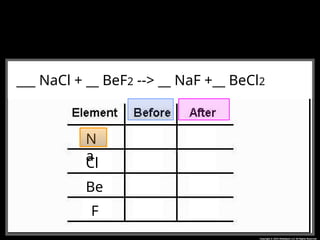
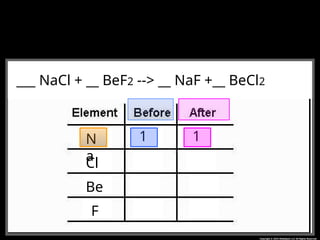
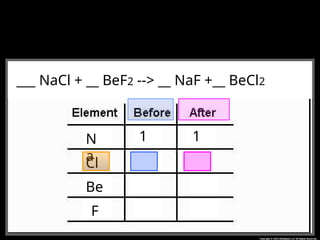
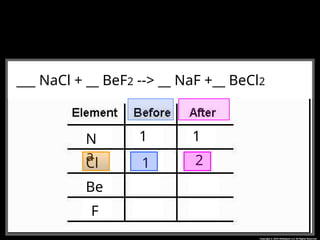
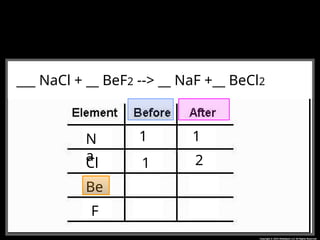
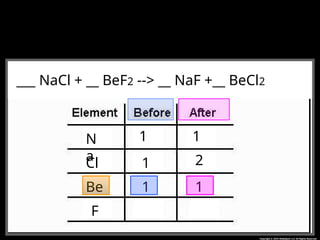
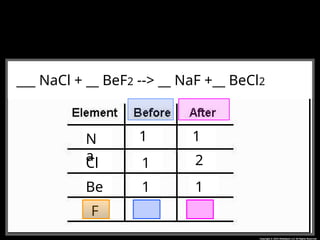
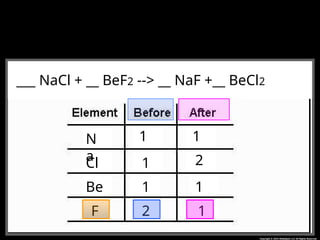
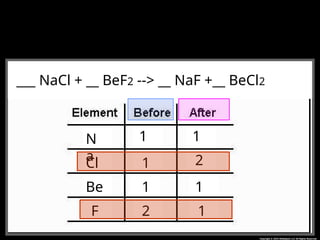
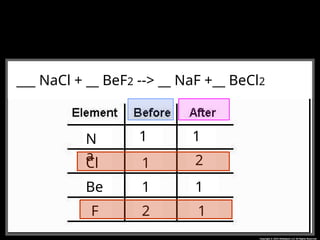
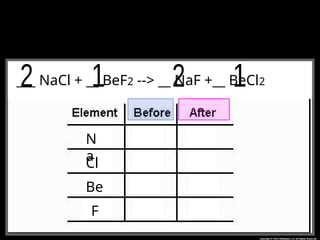
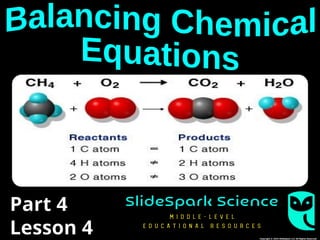
The document focuses on the concept of balancing chemical equations and the importance of understanding reactants and products in a chemical reaction. It emphasizes the law of conservation of mass, which states that matter cannot be created or destroyed, and provides guidelines for effectively balancing equations, including the use of coefficients. Additionally, it encourages maintaining an inventory chart of chemicals for accurate balancing.