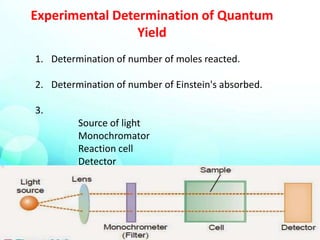
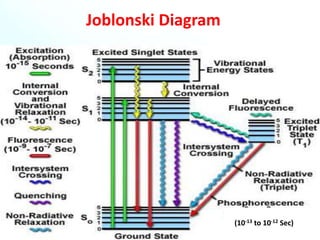
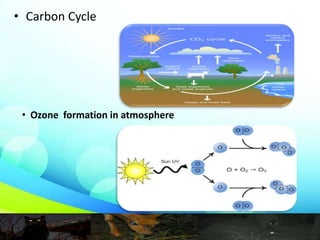
This document provides an overview of the topic of photochemistry. It discusses key concepts like quantum yield, which is a measure of reaction efficiency, and how it is experimentally determined. Diagrams like the Jablonski diagram are also mentioned. Specific photochemical processes covered include fluorescence, phosphorescence, chemiluminescence, and bioluminescence. Finally, some applications of photochemistry are listed, along with a bibliography for further reading.