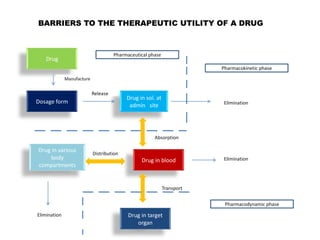
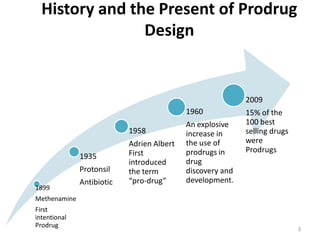
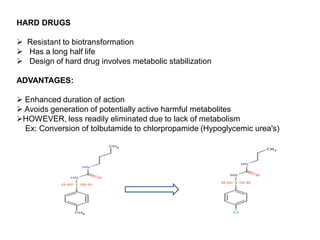
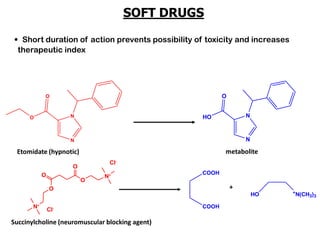
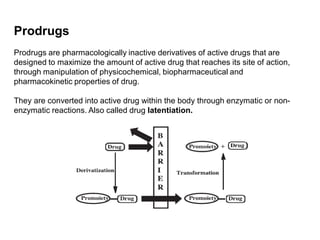
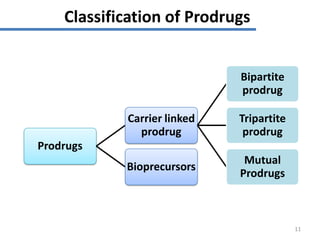
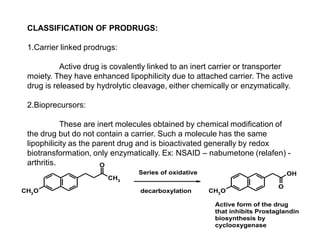
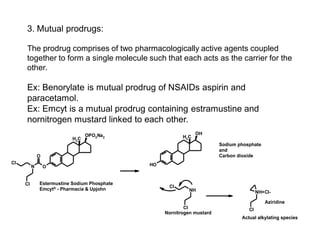
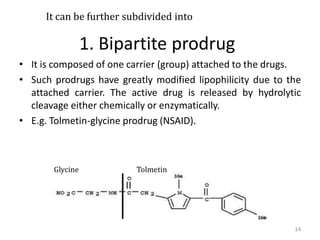
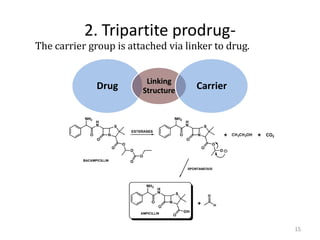
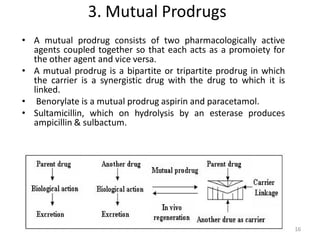
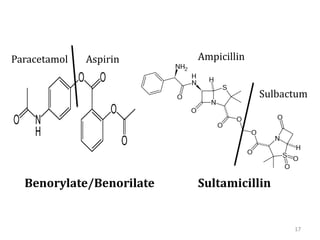
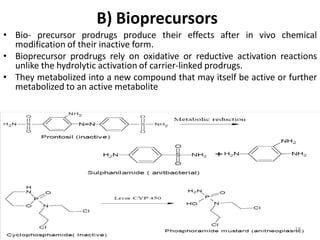
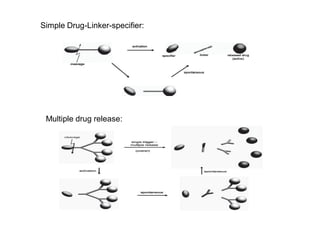
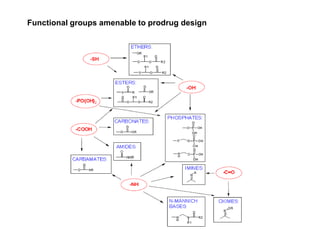
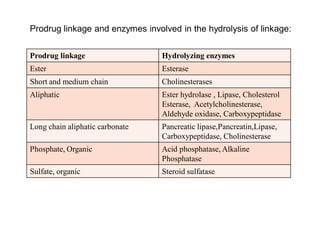
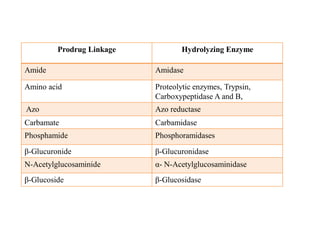
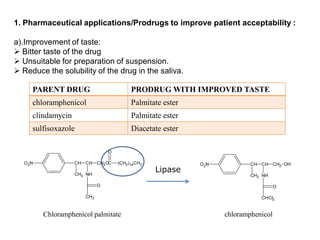
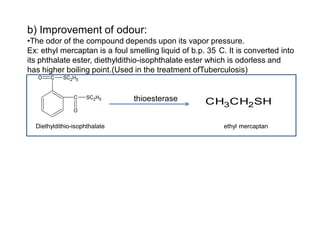
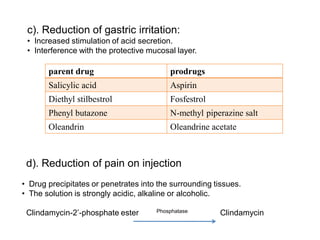
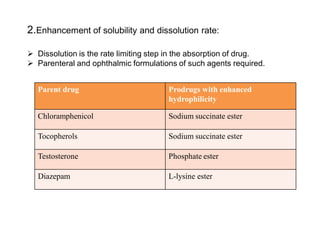
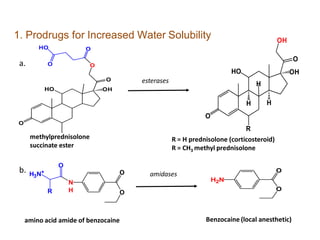
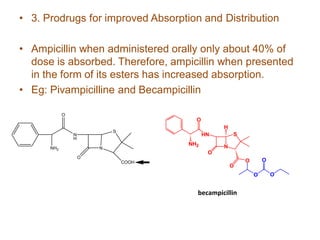
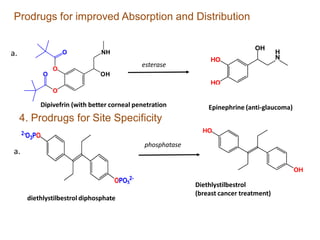
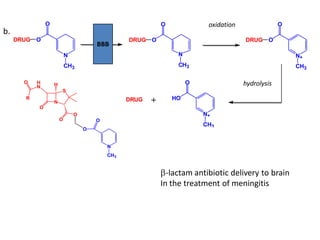
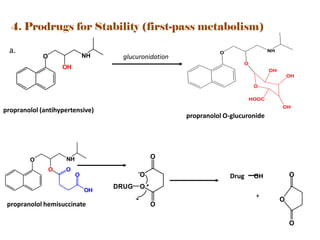
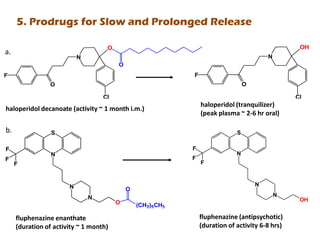
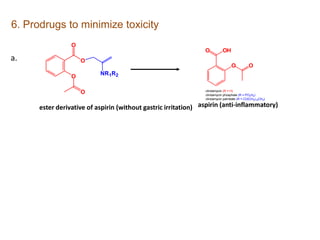
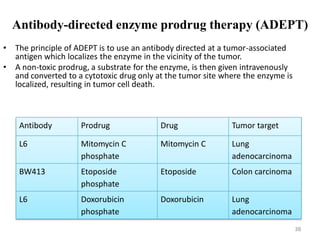
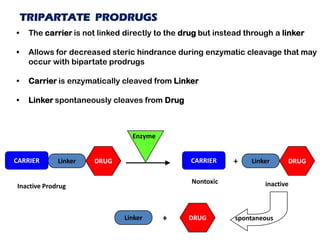
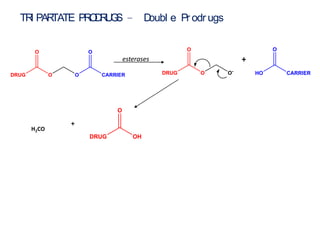
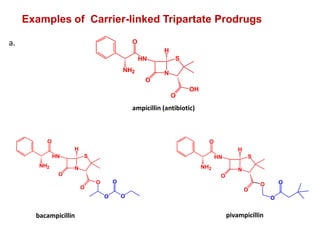
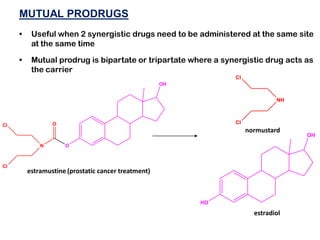
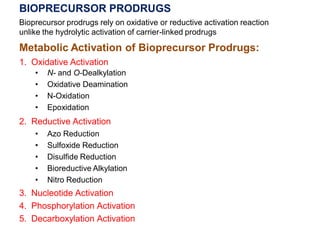
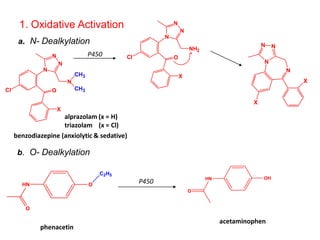
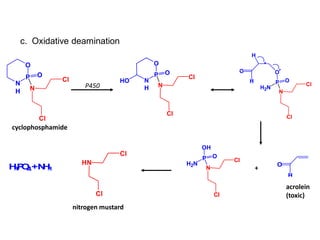
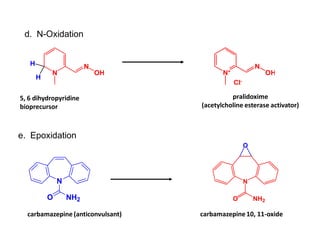
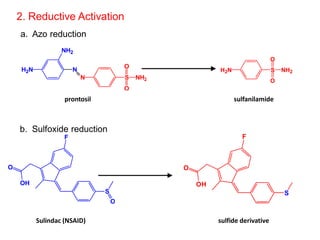
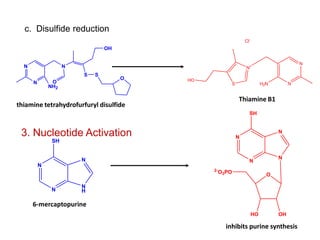
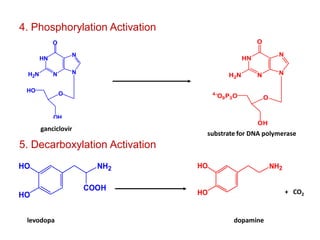
The document discusses prodrugs, which are pharmacologically inactive derivatives of active drugs designed to improve drug properties like solubility, absorption, and site-specific delivery. It covers basic prodrug concepts and classifications like carrier-linked prodrugs and bioprecursors. Approaches for prodrug design include using carriers, linkers, and multi-drug systems. Applications of prodrugs include improving patient acceptability by modifying taste, odor or irritation, enhancing solubility and dissolution for better absorption, and enabling site-specific or sustained drug delivery. The document provides examples of prodrug linkages and enzymes involved in their hydrolysis.