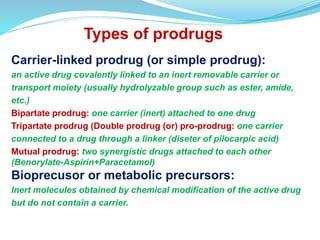
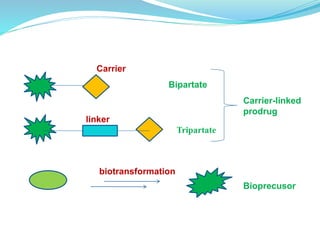
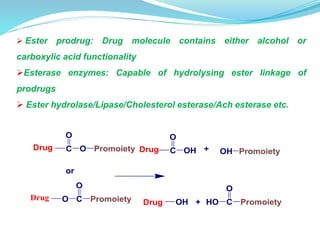
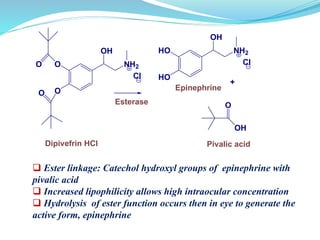
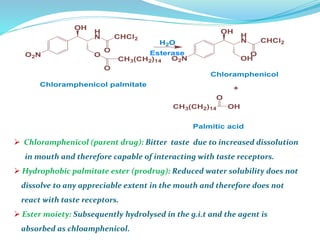
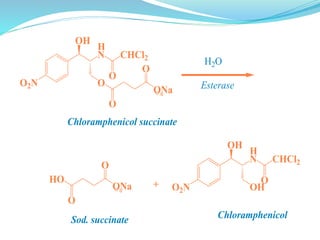
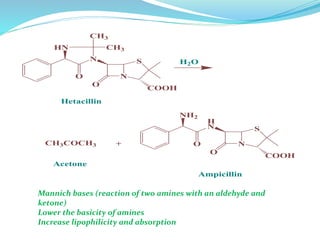
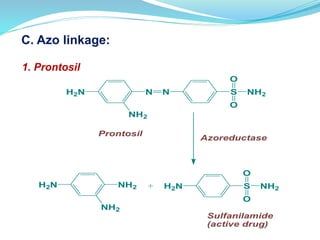
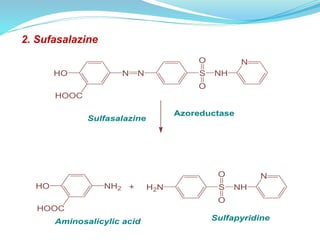
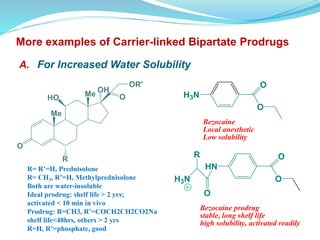
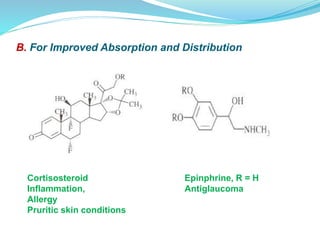
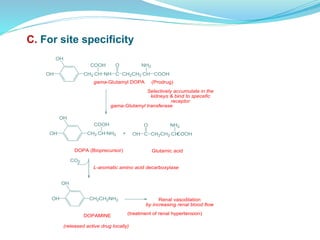
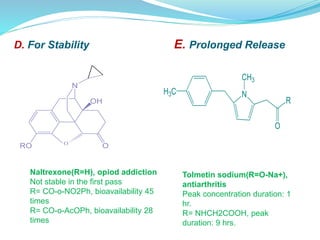
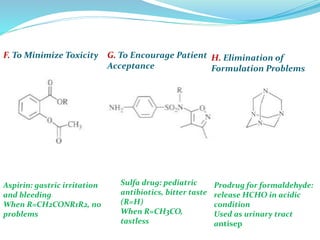
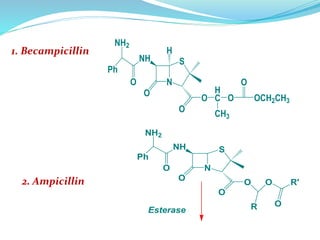
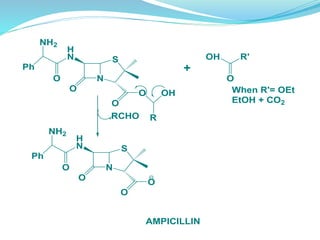
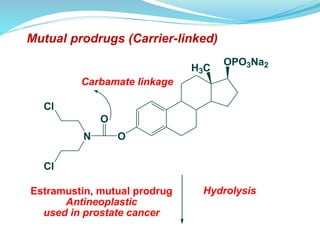
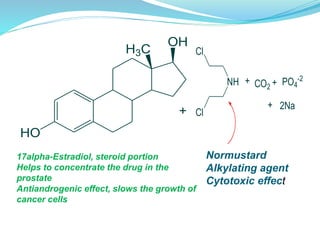
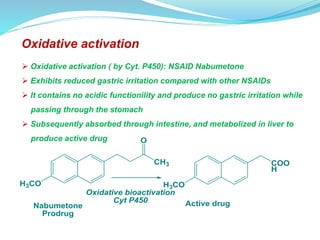
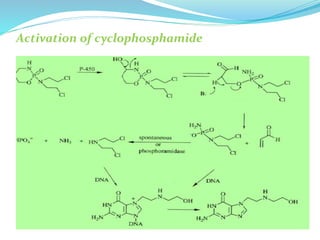
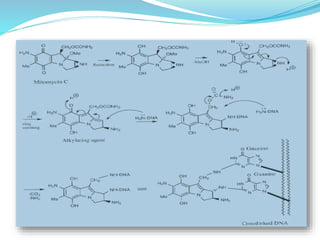
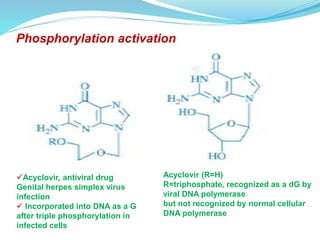
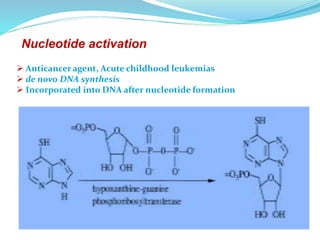
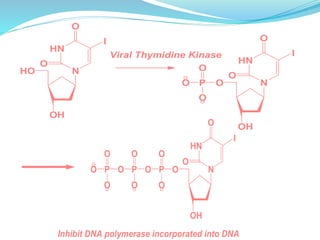
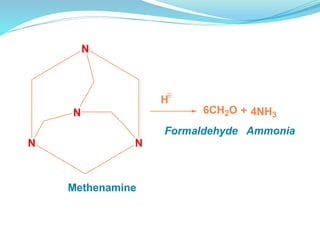
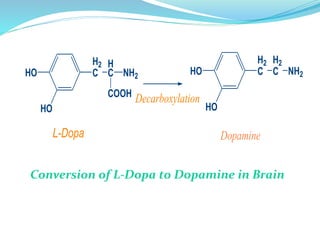
The document discusses prodrugs, which are pharmacologically inactive compounds that are converted to active drugs in the body. Prodrugs can undergo enzymatic or chemical conversion before, during, or after absorption. This conversion ideally occurs at the target site. The goals of prodrug design are to improve drug properties like taste, solubility, stability, and bioavailability, while ensuring metabolic byproducts are nontoxic. The document describes various types of prodrugs like carrier-linked, bipartate, tripartate, and bioprecursor prodrugs. It provides examples to illustrate how prodrugs can achieve outcomes like site-specific delivery and prolonged duration of action.