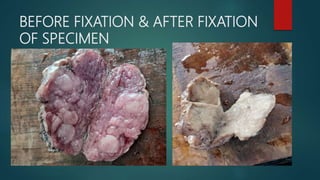
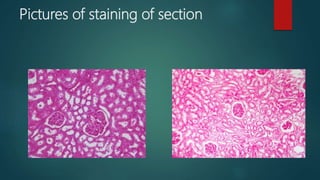
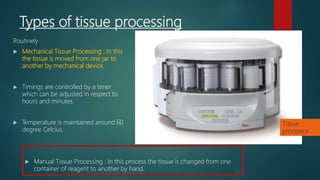
Histological techniques involve preparing tissue for microscopic examination through a series of procedures to retain the tissue's microscopic anatomy and allow for good staining. This involves fixing, processing, embedding, sectioning, and staining the tissue. The key steps are fixation to prevent deterioration, dehydration and clearing to infiltrate the tissue with wax, sectioning thin slices, and staining, most commonly with hematoxylin and eosin, to highlight structures. The goal is to represent the tissue's structure as closely as possible to its in vivo state.