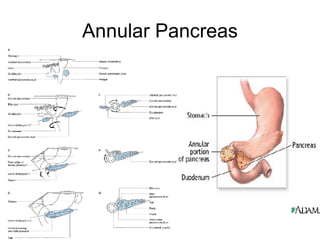
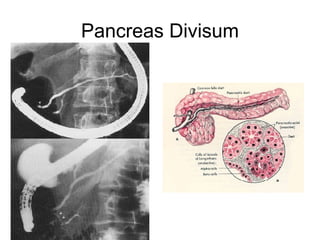
1. The document discusses several diseases of the pancreas including congenital problems like annular pancreas and pancreas divisum.
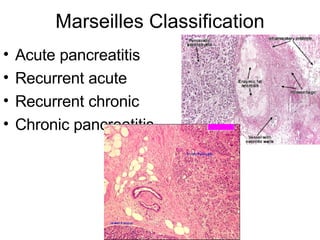
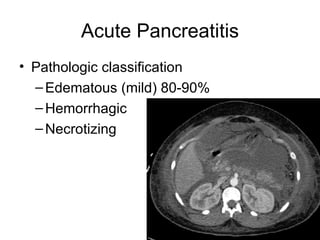
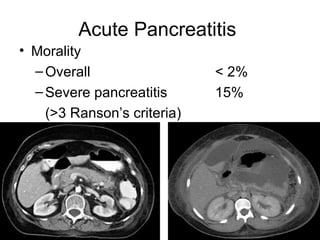
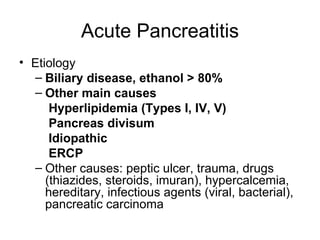
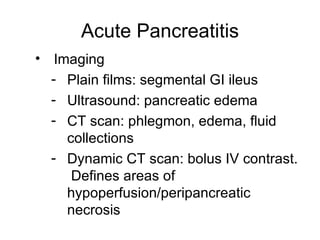
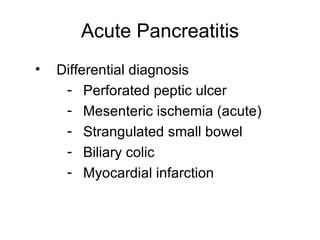
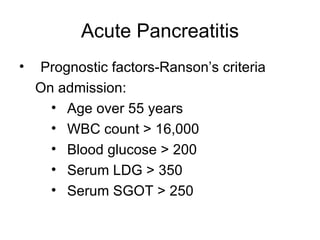
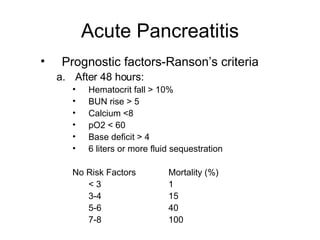
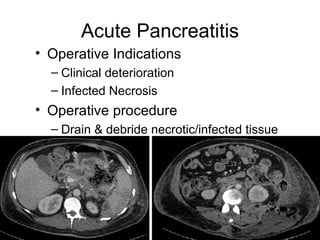
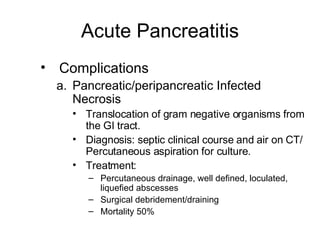
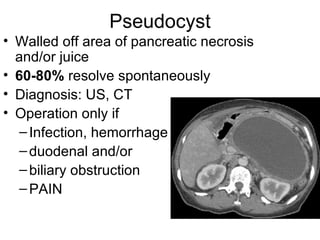
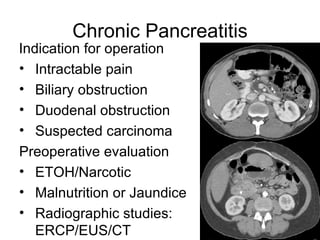
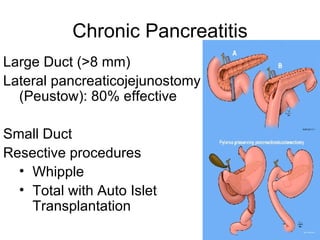
2. It covers acute and chronic pancreatitis, their causes, symptoms, prognosis, and treatment options including supportive care and surgery.
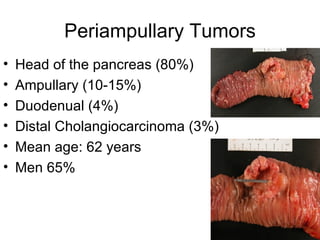
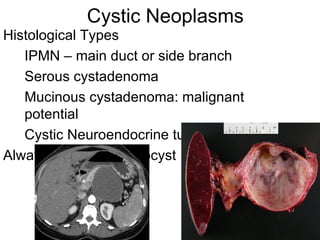
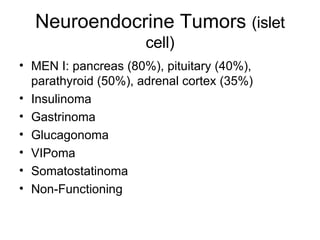
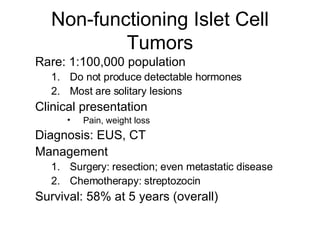
3. The document also discusses several types of pancreatic tumors and cysts, their diagnosis and treatment through surgery or other palliative options. It provides details on specific neuroendocrine tumors.