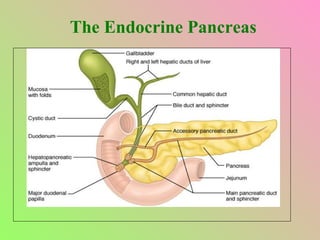
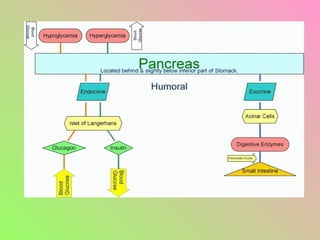
The document summarizes key aspects of the endocrine pancreas and its hormones. It discusses:
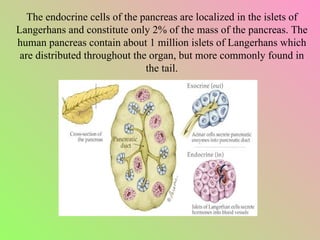
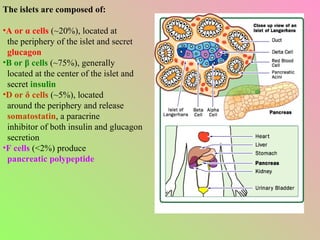
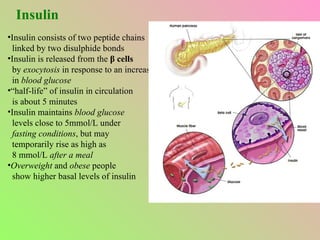
- The islets of Langerhans contain alpha, beta, and delta cells that secrete glucagon, insulin, and somatostatin.
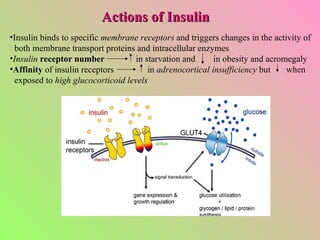
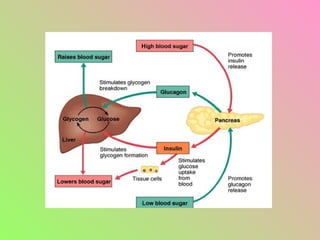
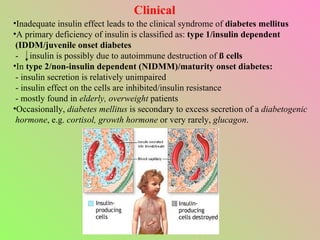
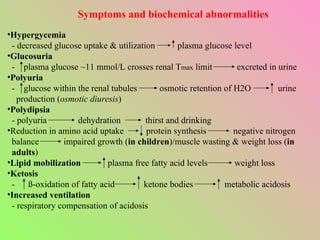
- Insulin regulates blood glucose levels through effects on glucose and lipid metabolism. Insulin secretion is stimulated by high blood glucose.
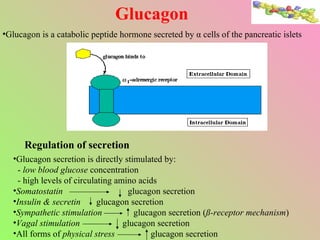
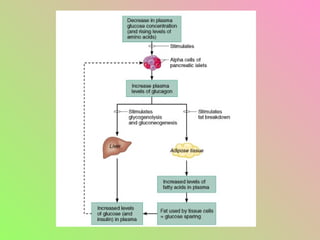
- Glucagon opposes insulin's effects and raises blood glucose through hepatic glycogenolysis and gluconeogenesis. It is secreted in response to low blood glucose.
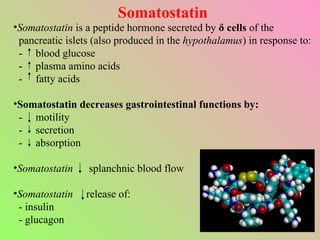
- Somatostatin is secreted by delta cells and decreases secretion of insulin and glucagon. It also regulates gastrointestinal functions.