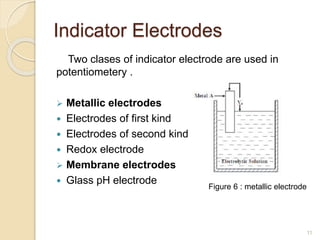
Potentiometry is a technique used to determine the concentration of a solute in solution by measuring the potential difference between two electrodes using a voltmeter. It works based on measuring the voltage between a reference electrode, which has a constant potential, and an indicator electrode, whose potential changes based on the concentration of ions in solution. Common reference electrodes include the standard hydrogen electrode and the silver/silver chloride electrode. Indicator electrodes can be metallic electrodes that respond to specific cations or anion-selective electrodes like glass or solid state membranes. Potentiometry finds applications in clinical chemistry, environmental analysis, agriculture, and other industries.