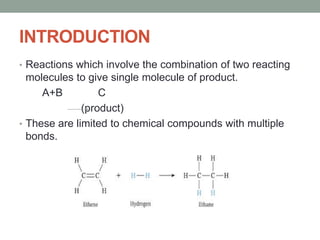
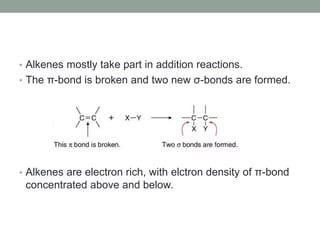
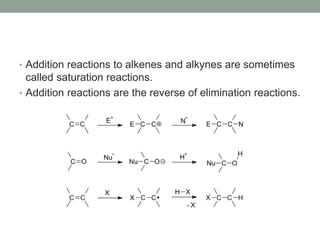
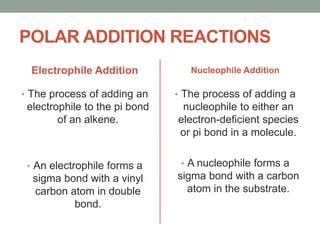
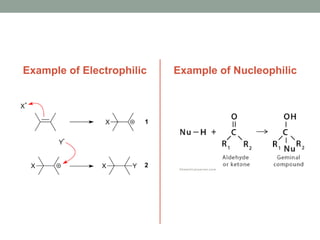
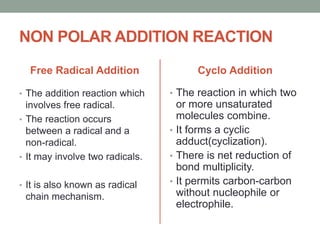
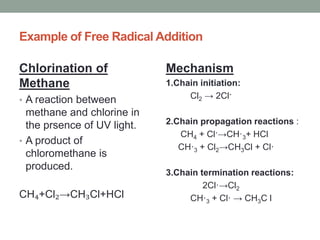
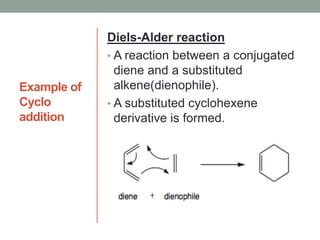
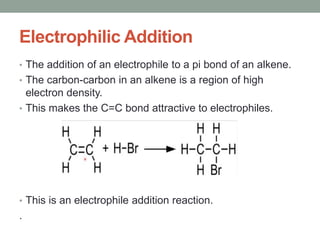
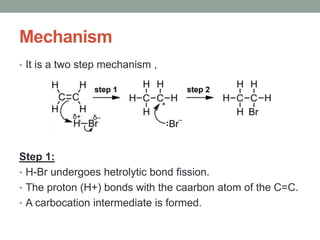
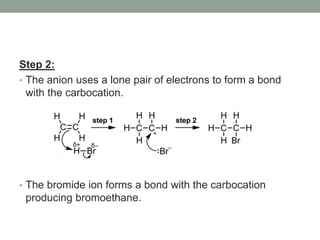
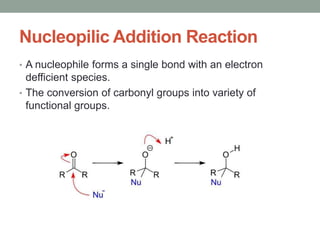
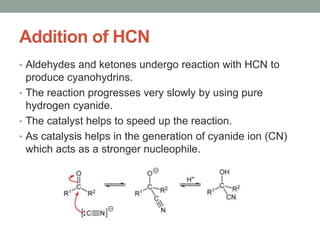
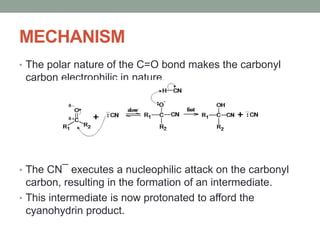
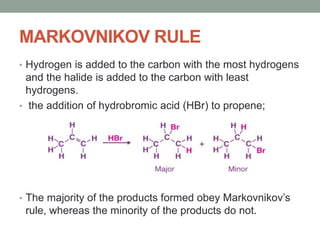
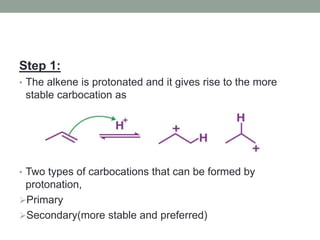
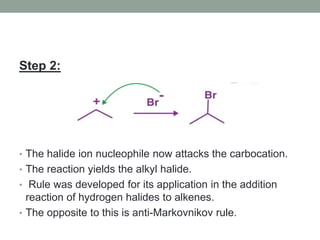
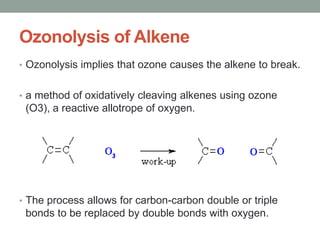
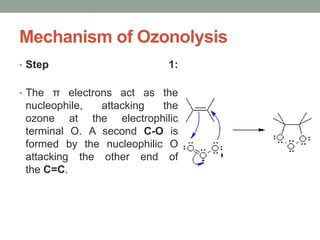
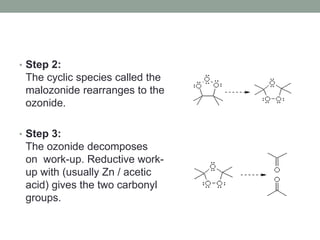
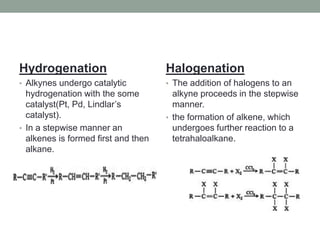
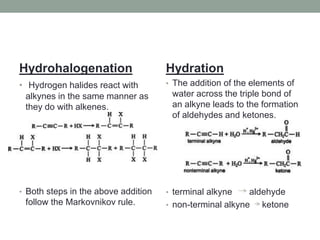
Este documento aborda las reacciones de adición, que implican la combinación de dos moléculas para formar un solo producto, especialmente en compuestos con enlaces múltiples como alquenos y alquinos. Se describen varios tipos de reacciones de adición, incluyendo la adición electrofílica y nucleofílica, así como las reglas de Markovnikov y el ozonólisis de alquenos. Además, se analizan los mecanismos de estas reacciones y sus aplicaciones en la química orgánica.





![REFERENCES
1. Morrison, R. T.; Boyd, R. N. (1983). Organic
Chemistry(4th ed.). Boston: Allyn and Bacon.
2. March, Jerry; (1985). Advanced Organic Chemistry
reactions, mechanisms and structure (3rd ed.). New York:
John Wiley & Sons.
3. Fleming, Ian (2010). Molecular orbitals and organic chemical
reactions. New York: Wiley.
4. Myles W. Smith; Phil S. Baran (2015-08-28). "As simple as
[2+2]". Science 349 (6251): 925–926.
5. Hein, Sara M. (June 2006). "An Exploration of a
Photochemical Pericyclic Reaction Using NMR
Data". Journal of Chemical Education. 83 (6): 940–942.](https://image.slidesharecdn.com/additionreaction-220709174403-81da5a2d/85/Addition-Reaction-pptx-28-320.jpg)
