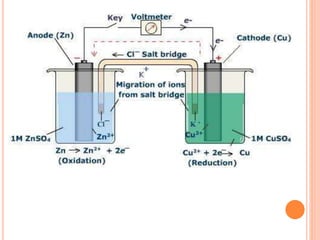
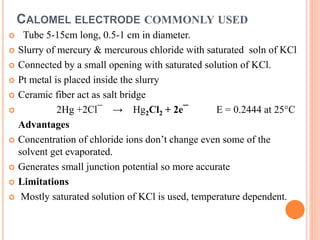
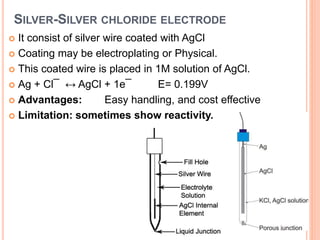
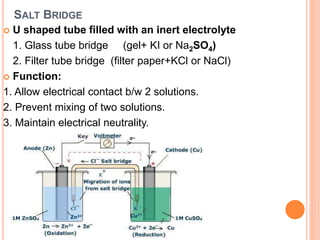
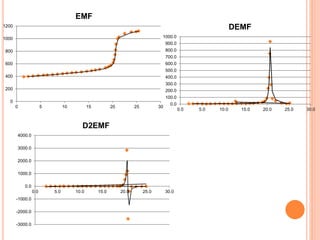
The document provides a comprehensive overview of potentiometry, detailing its historical development, methods, and applications in electroanalytical chemistry. It explains the principle of measuring potential differences between electrodes, various types of electrodes used (including reference and indicator electrodes), and different forms of titration techniques. Additionally, it discusses the applications of potentiometry across multiple industries including environmental monitoring, pharmaceuticals, and food quality analysis.