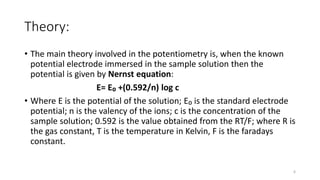
Potentiometry is an analytical technique that measures the potential of electrochemical cells without drawing current. It involves using a reference electrode with a known potential and an indicator electrode whose potential varies with analyte concentration. The cell potential is measured and related to concentration using the Nernst equation. Common reference electrodes include the standard hydrogen electrode and saturated calomel electrode. Glass membrane and ion-selective electrodes are often used as indicator electrodes to detect specific ions like hydrogen or fluoride ions. Potentiometry finds applications in clinical analysis, environmental monitoring, and titration experiments.