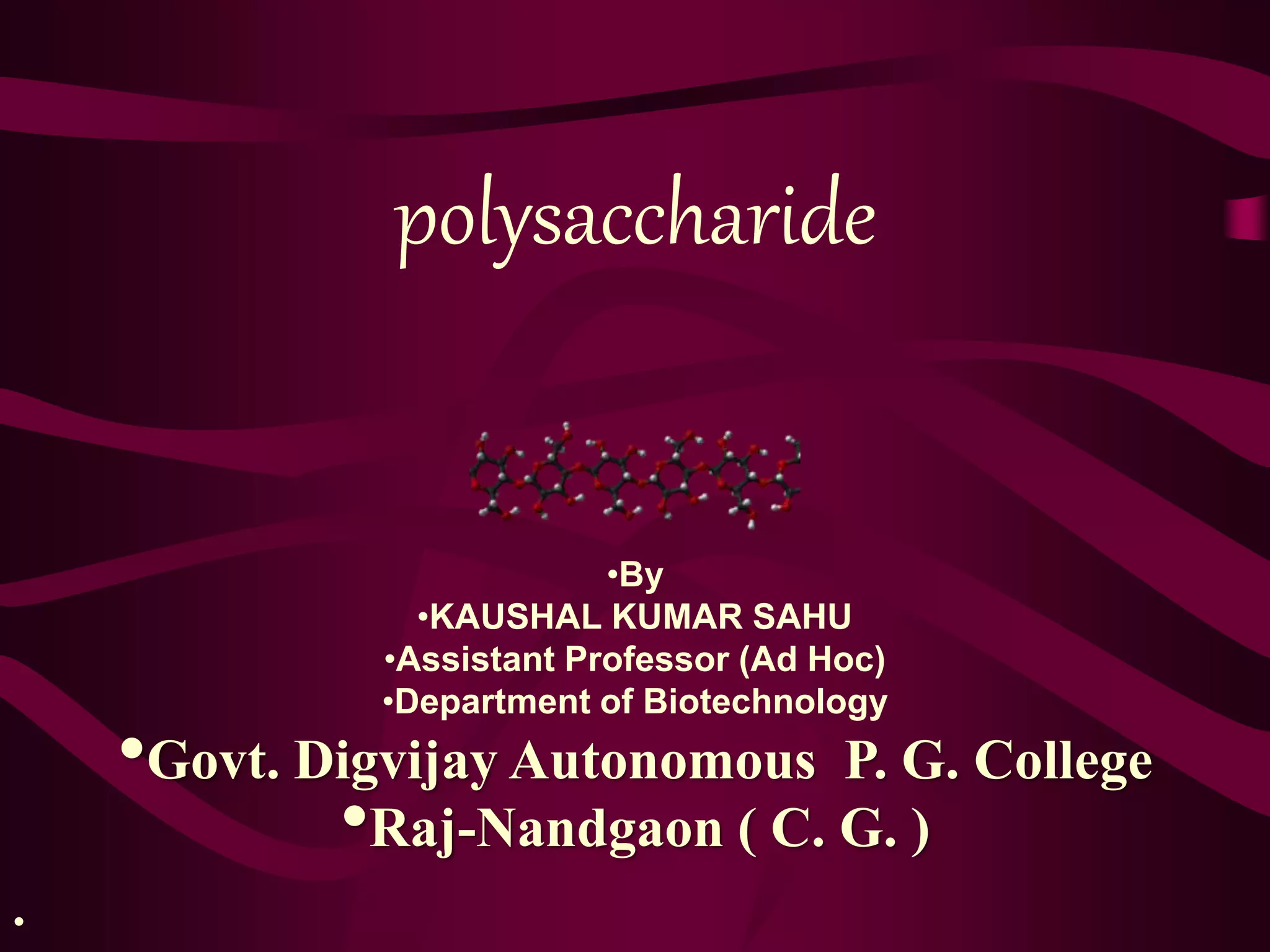
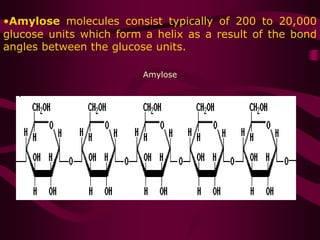
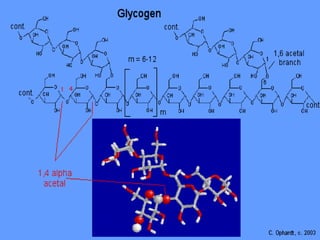
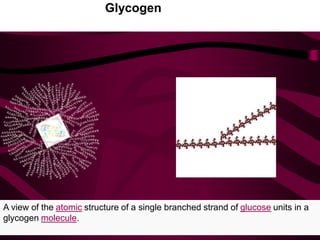
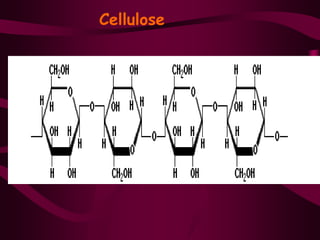
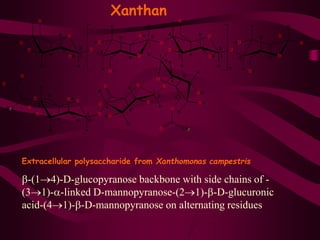
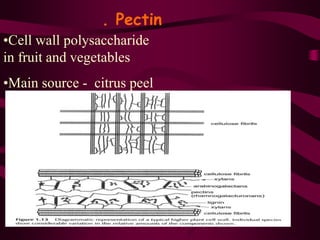
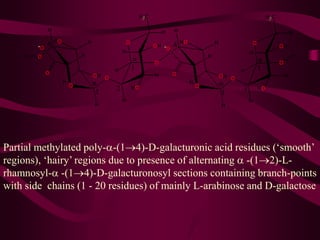
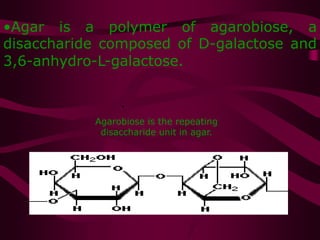
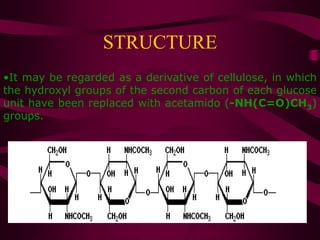
The document discusses polysaccharides, classifying them into three types based on chemical composition: monosaccharides, oligosaccharides, and polysaccharides, while highlighting their sources and functions. It elaborates on the structure and properties of important polysaccharides such as starch, glycogen, cellulose, xanthan, pectin, agar, inulin, and chitin, detailing their roles in energy storage, structural integrity, and industrial applications. Additionally, the document addresses the health benefits and physiological effects of polysaccharides, including immune regulation and potential anti-diabetic properties.