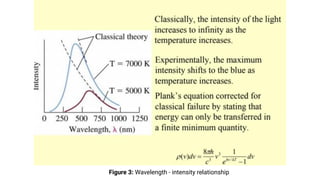
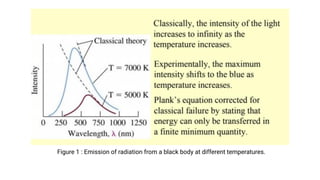
The document provides an introduction to quantum mechanics and its relevance in chemistry, covering topics such as black body radiation, the photoelectric effect, Planck's radiation law, and the Compton effect. It explains how classical mechanics differs from quantum mechanics, emphasizing the need for quantum theories to explain phenomena that classical theories cannot. Key concepts are illustrated with equations and examples, highlighting the particle nature of light and energy quantization.








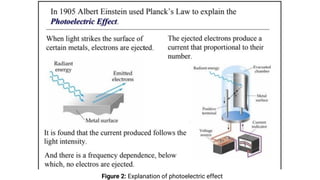

![● Photoelectric
effect
remaining energy will be imparted to ejected
electron to give it certain velocity u( i.e, K. E=1/2
mu2
). Einstein applying quantum theory showed
that,
hv = ∅ +1/2mu2
_______1
where ∅ =threshold energy of metal and 1/2mu2
is
K. E imparted to ejected electron.
∅ = hv0
_________2
Substituting the equation 2 in equation 1
1/2 mu2
= h(v-v0)
) ______[Einstein’s
Photoelectric Effect]](https://image.slidesharecdn.com/introductionquantummechanicschemistry-210410203519/85/Introduction-quantum-mechanics-chemistry-14-320.jpg)