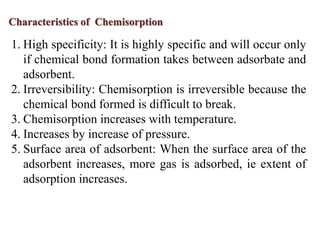
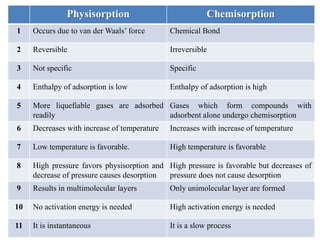
This document discusses different types of adsorption - physisorption and chemisorption - and how they differ. Physisorption involves weak van der Waals forces between adsorbate and adsorbent molecules, while chemisorption involves chemical bond formation. It also discusses isotherm models like the Langmuir and Freundlich isotherms that describe the relationship between amount of gas adsorbed and pressure or concentration. Finally, it mentions that adsorption can change the work function of materials by altering charge distribution and dipole formation at the surface.