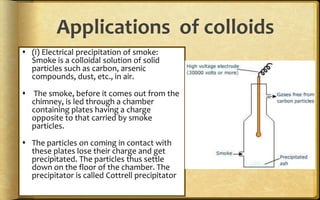
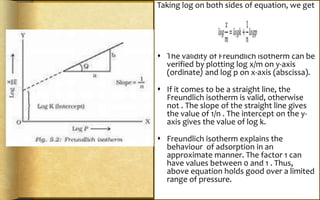
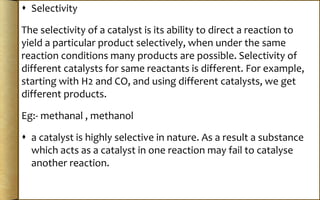
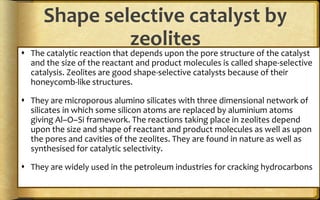
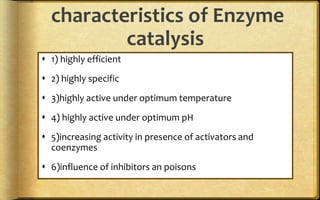
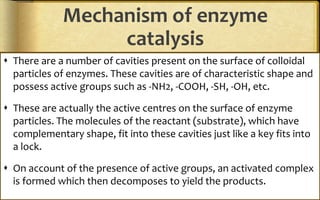
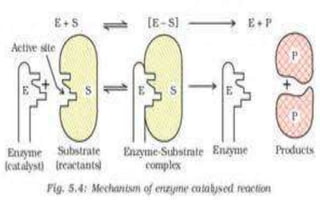
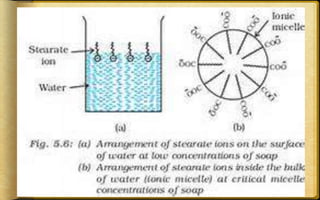
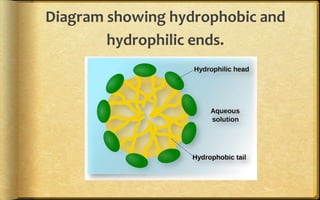
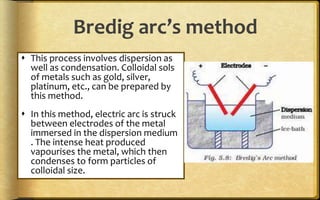
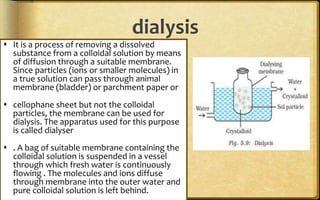
The document discusses adsorption, which is the accumulation of molecular species at the surface of a solid or liquid rather than in the bulk. The substance accumulating at the surface is called the adsorbate, and the material it accumulates on is the adsorbent. Adsorption can occur with gases accumulating on charcoal, dyes on animal charcoal, or water molecules on silica gel. It is influenced by factors like temperature, pressure, surface area, and the strength of interaction between adsorbate and adsorbent molecules. There are two main types - physical adsorption due to weak van der Waals forces, and chemical adsorption where chemical bonds form. Adsorption finds applications in areas





























![ Similarly, in the coagulation of a positive sol, the flocculating
power is in the order: [Fe(CN)6]4– > PO43– > SO42– > Cl–
The minimum concentration of an electrolyte in millimoles per
litre required to cause precipitation of a sol in two hours is
called coagulating value. The smaller the quantity needed, the
higher will be the coagulating power of an ion.](https://image.slidesharecdn.com/surfacechemistryppt-170825145553/85/Surfacechemistryppt-62-320.jpg)