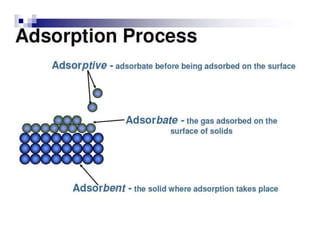
Gas adsorption techniques are used to measure the specific surface area, porosity, and pore size distribution of materials. The technique involves adsorbing gas molecules such as nitrogen onto the surface of a solid and determining the quantity adsorbed at different pressures to generate an adsorption isotherm. The isotherm is then analyzed using the BET equation to calculate the specific surface area by determining the amount of gas required for monolayer coverage. Other analyses of the isotherm using techniques such as the Kelvin equation allow determining the pore size distribution. Gas adsorption is a common, established technique for characterizing porous materials that can measure pore sizes from 0.4-50 nm.