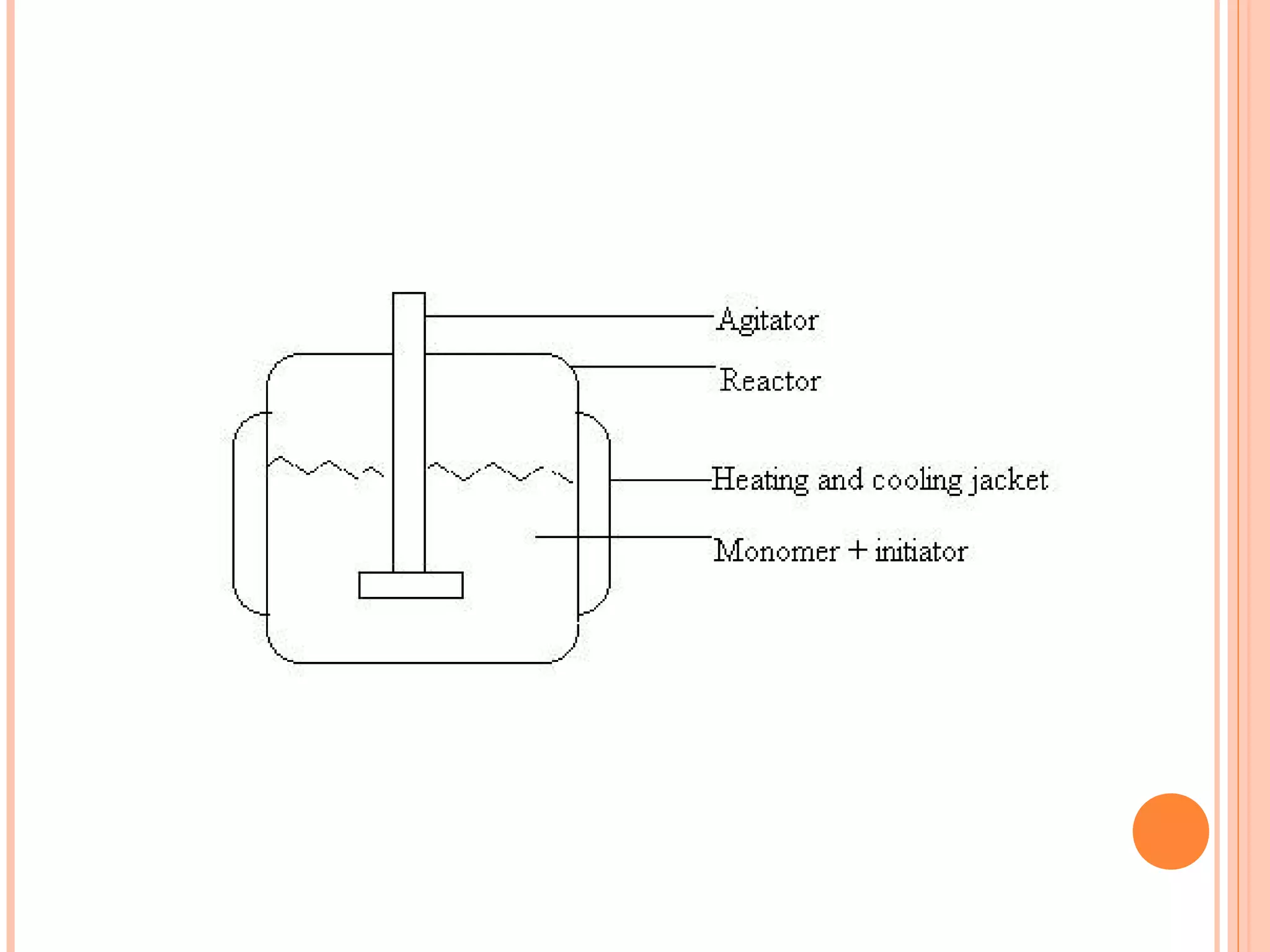
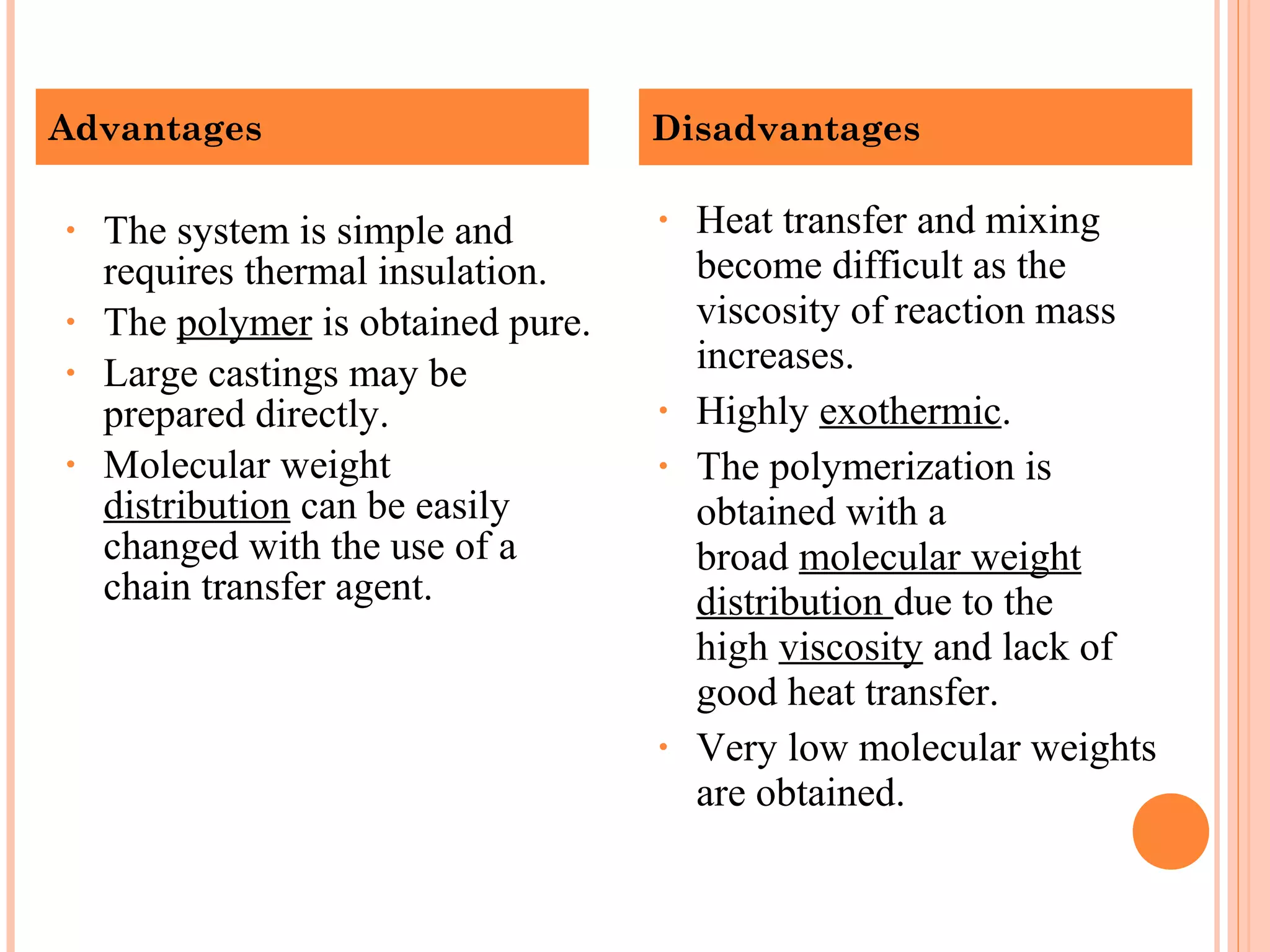
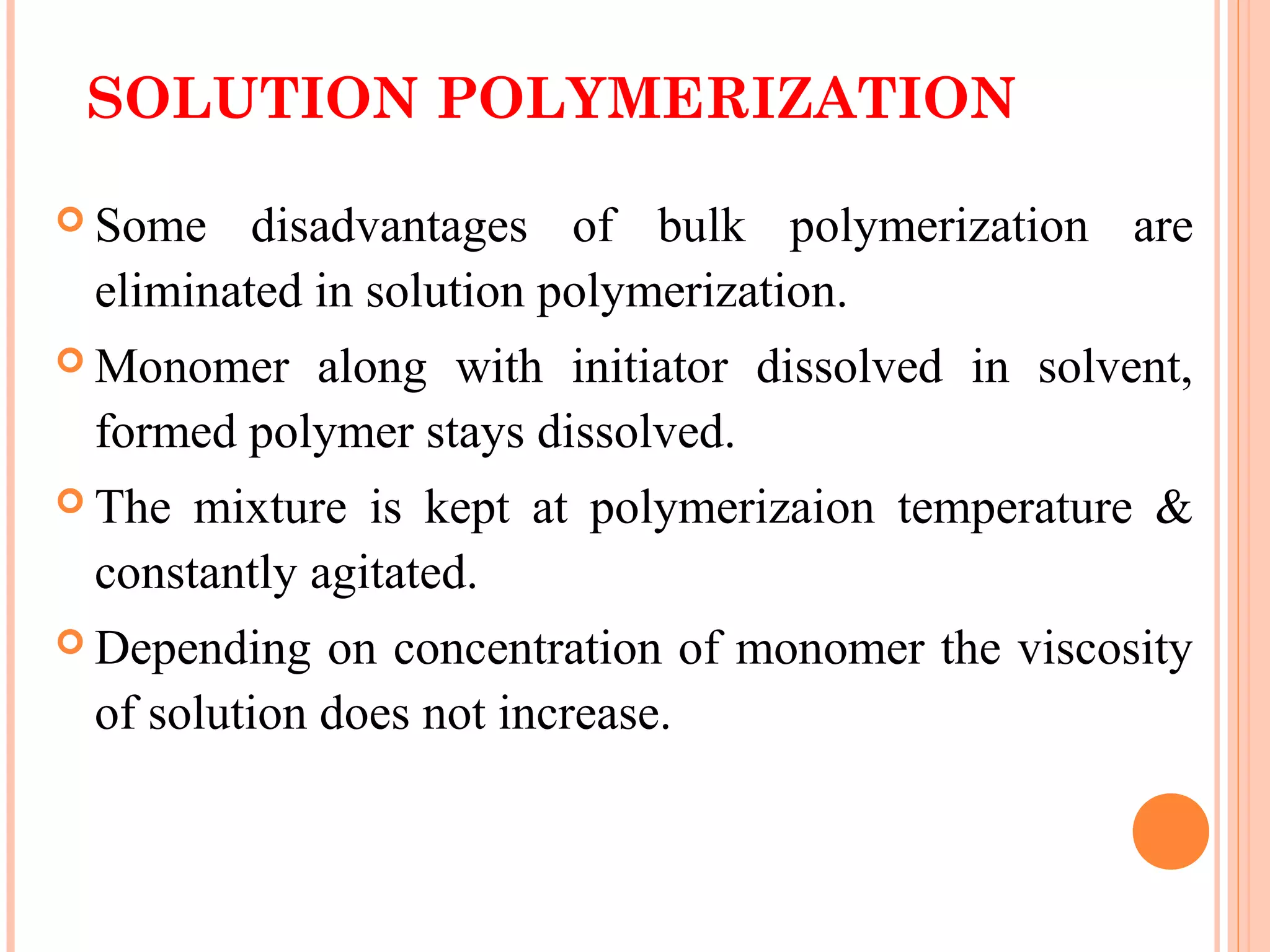
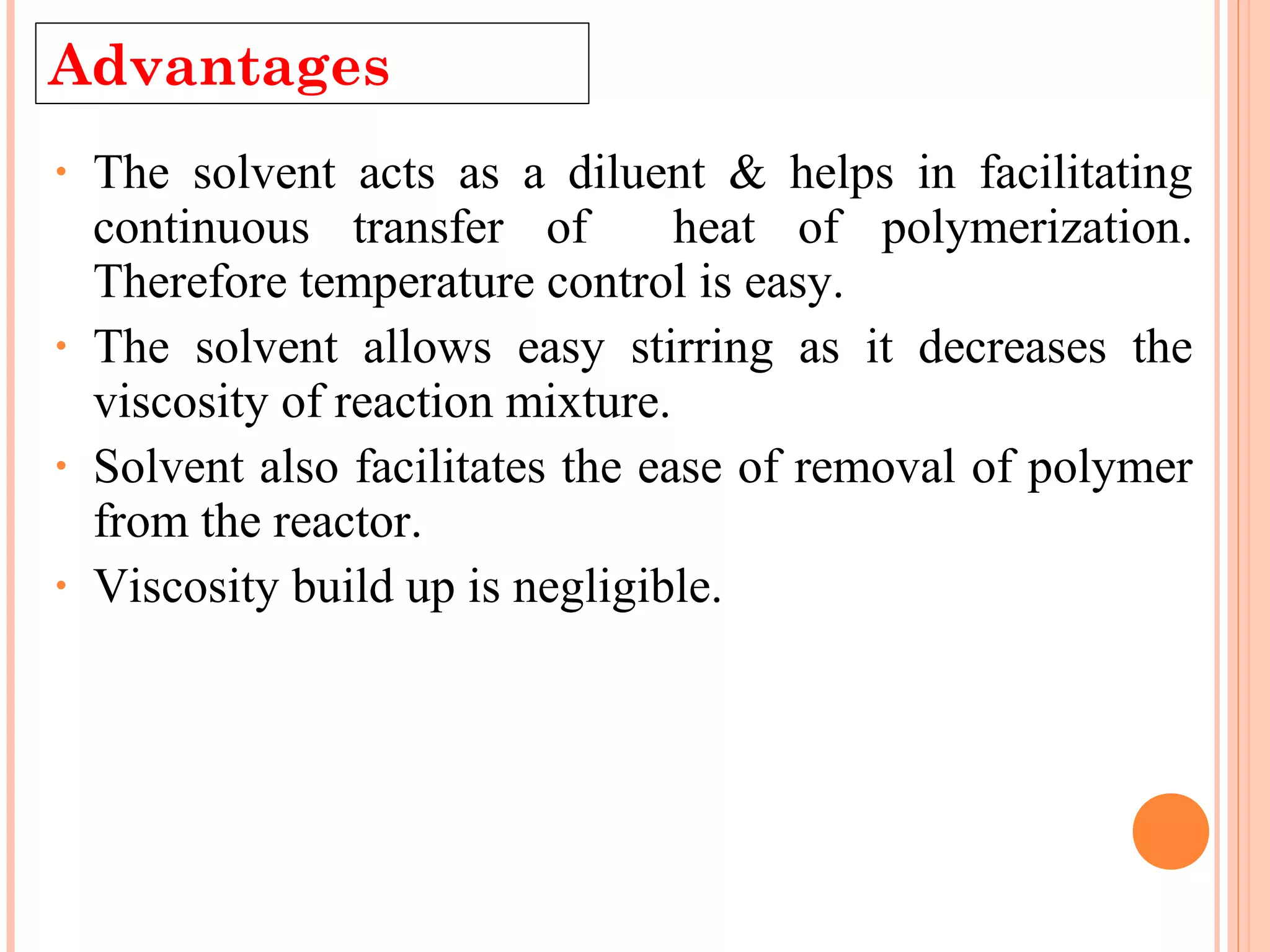
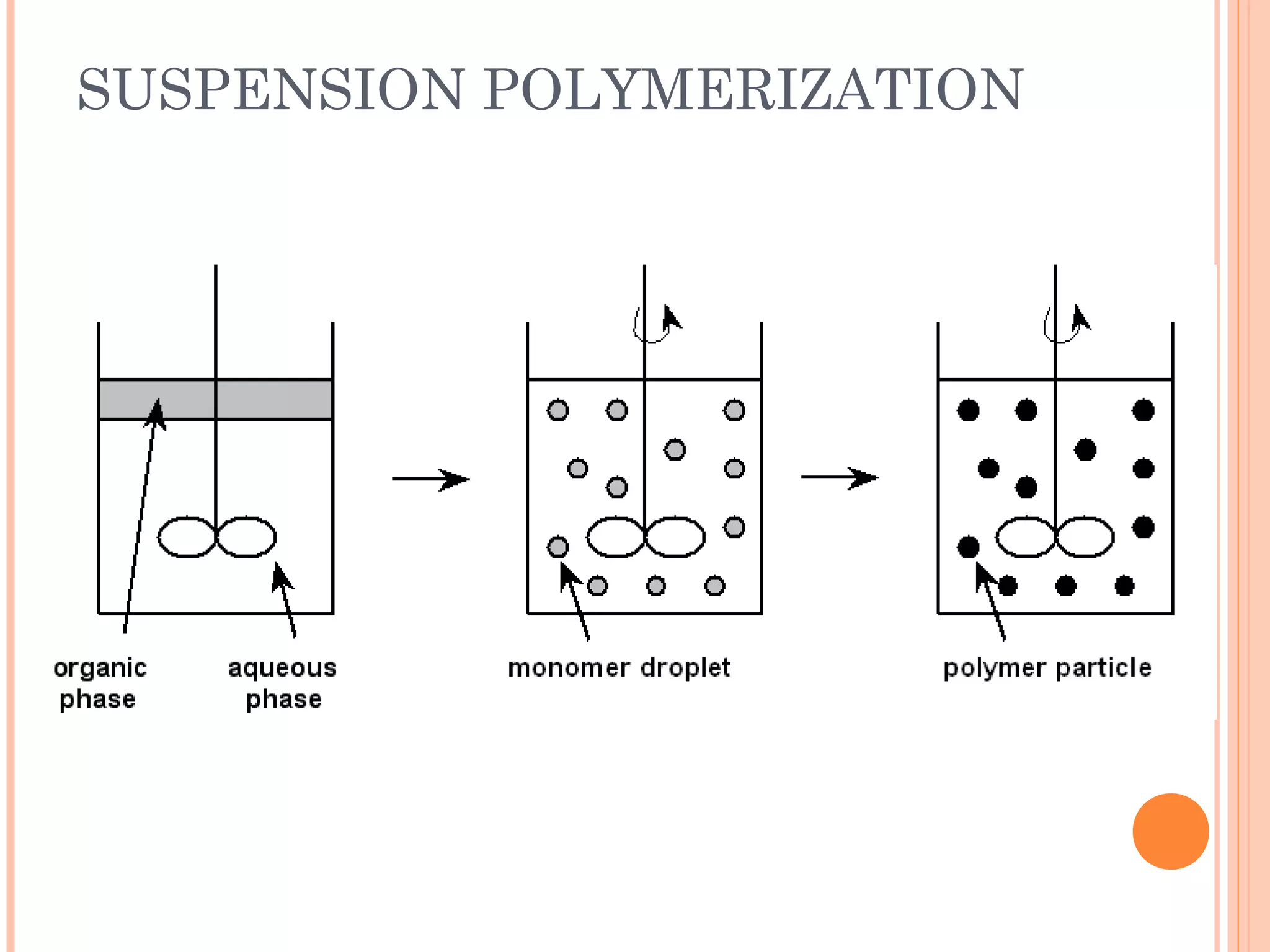
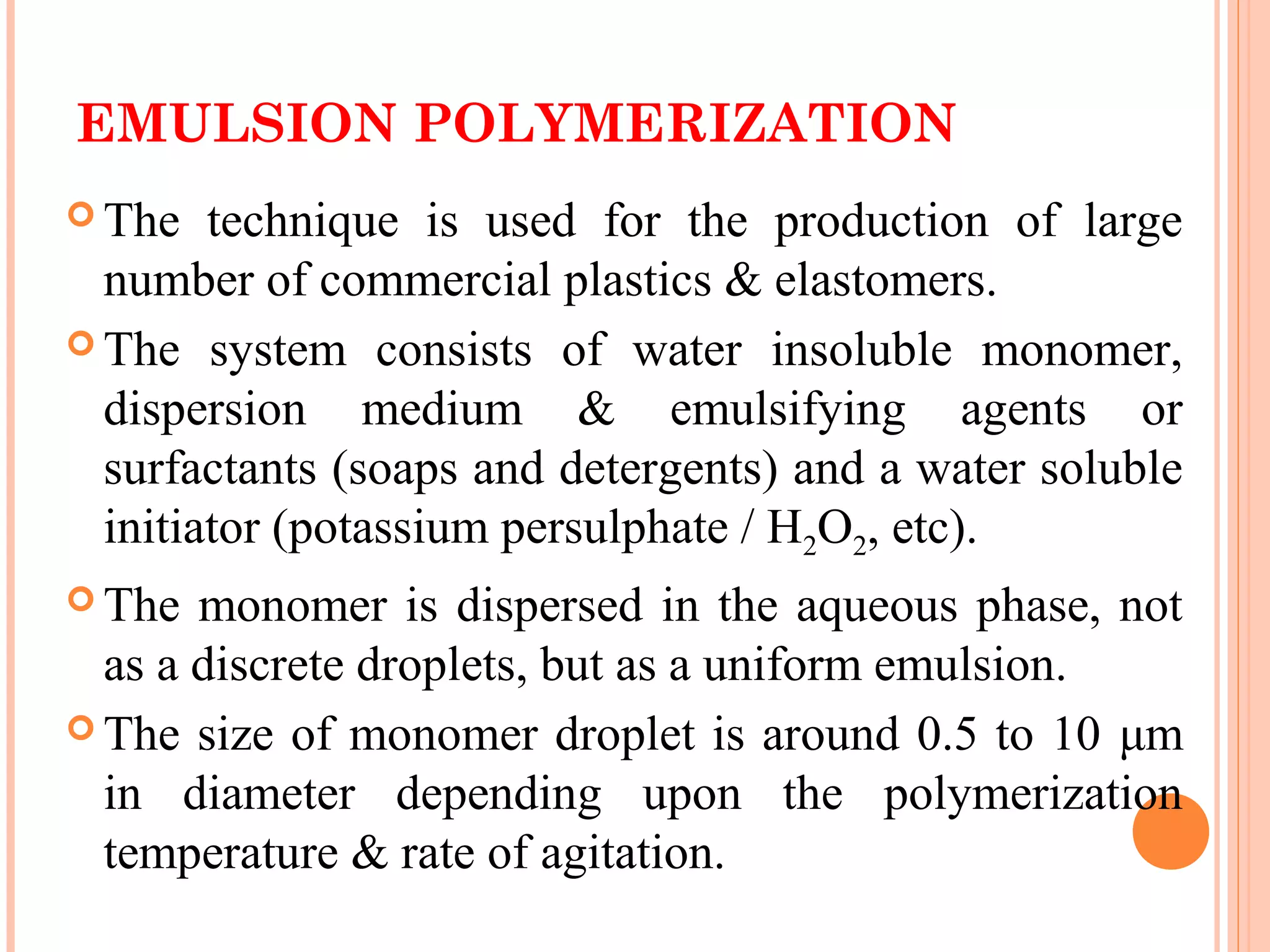
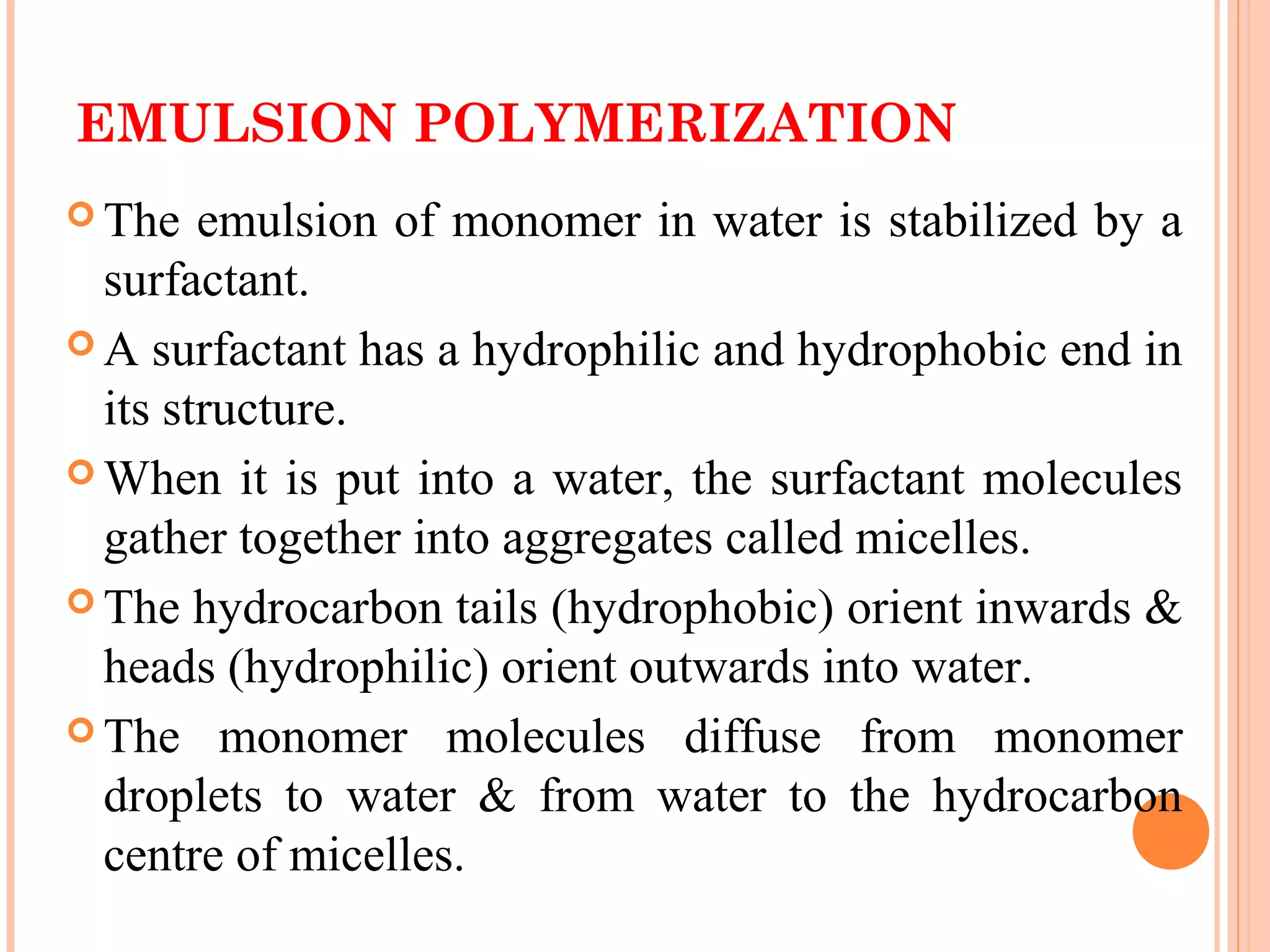
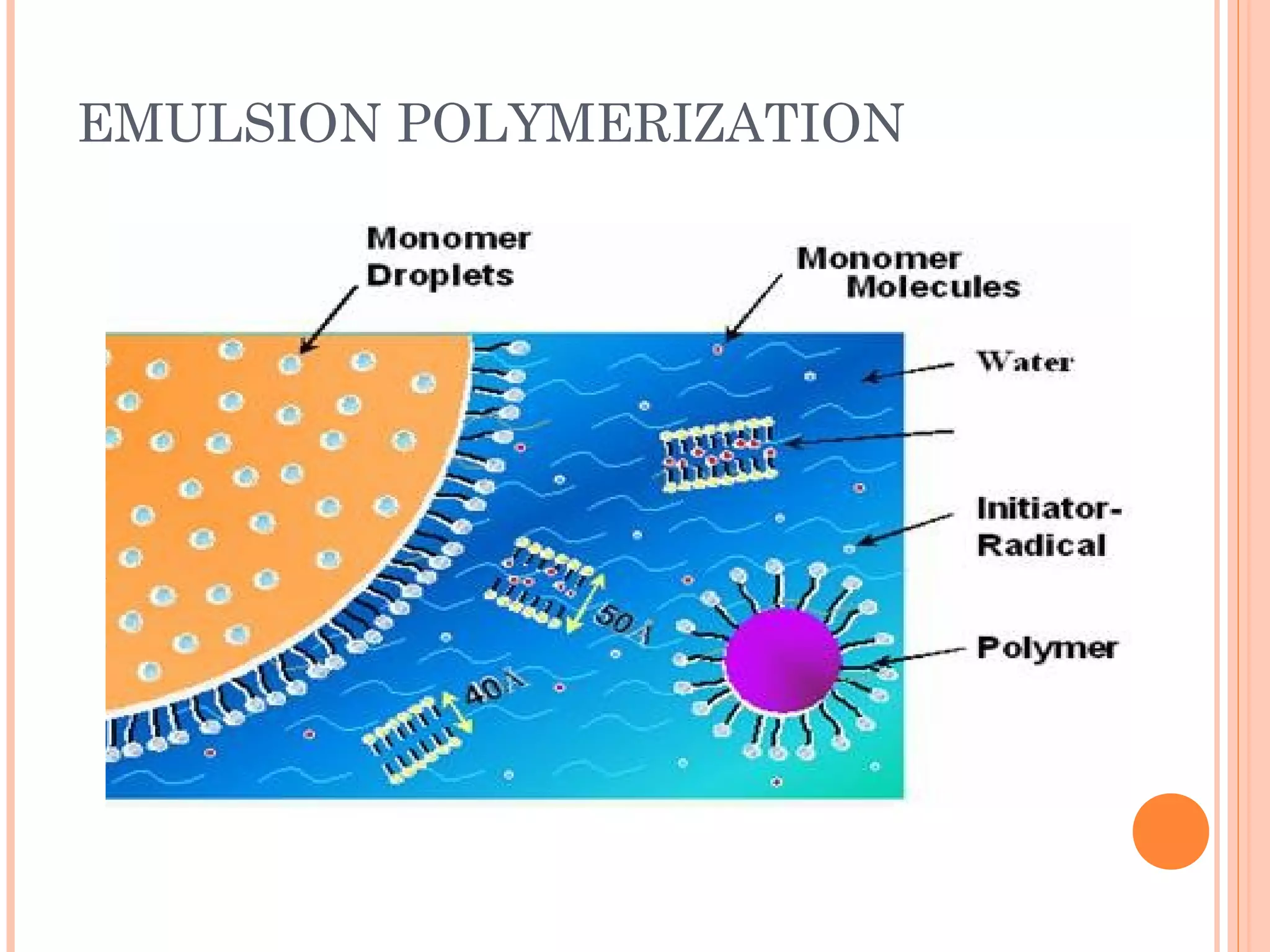
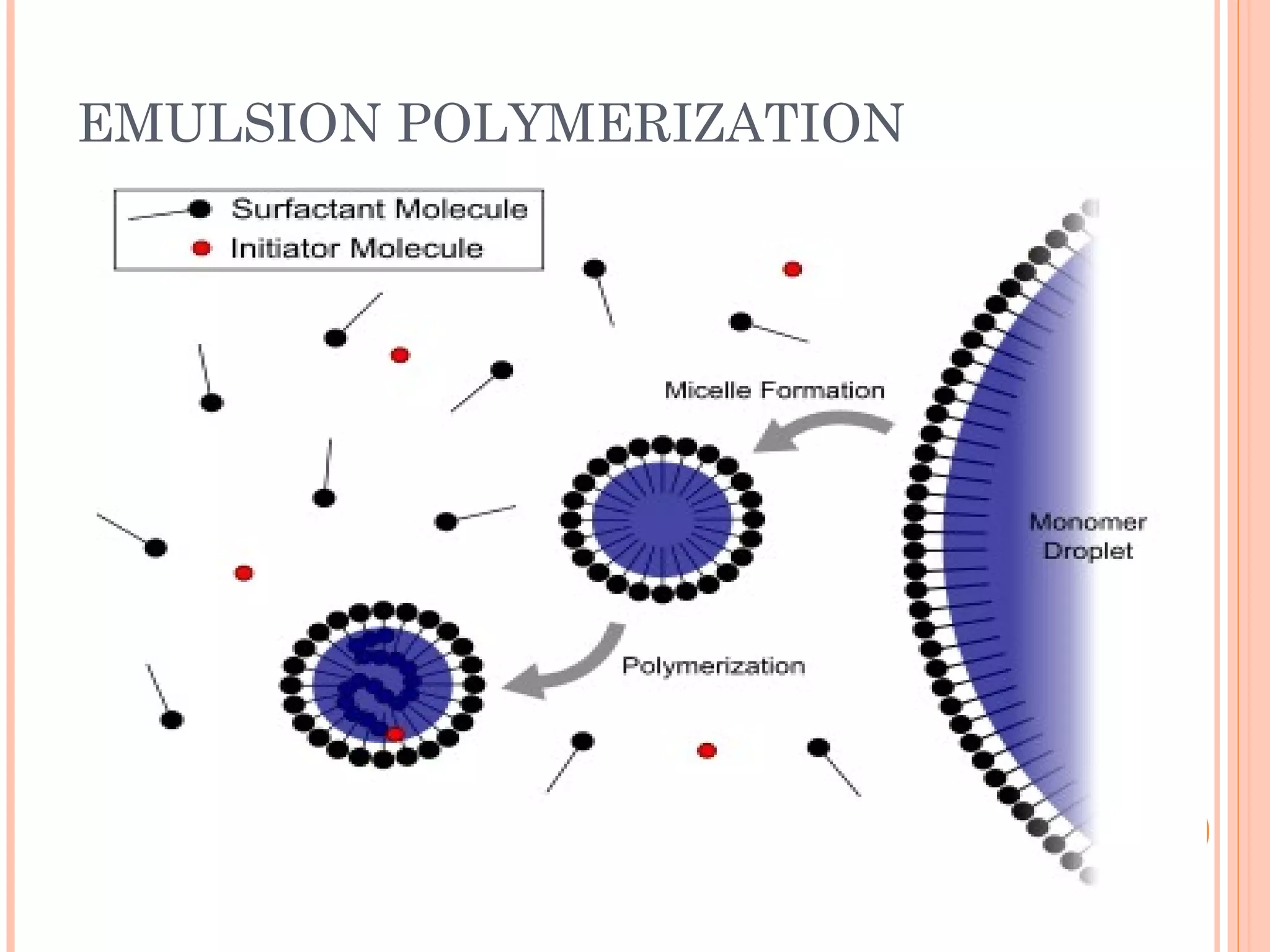
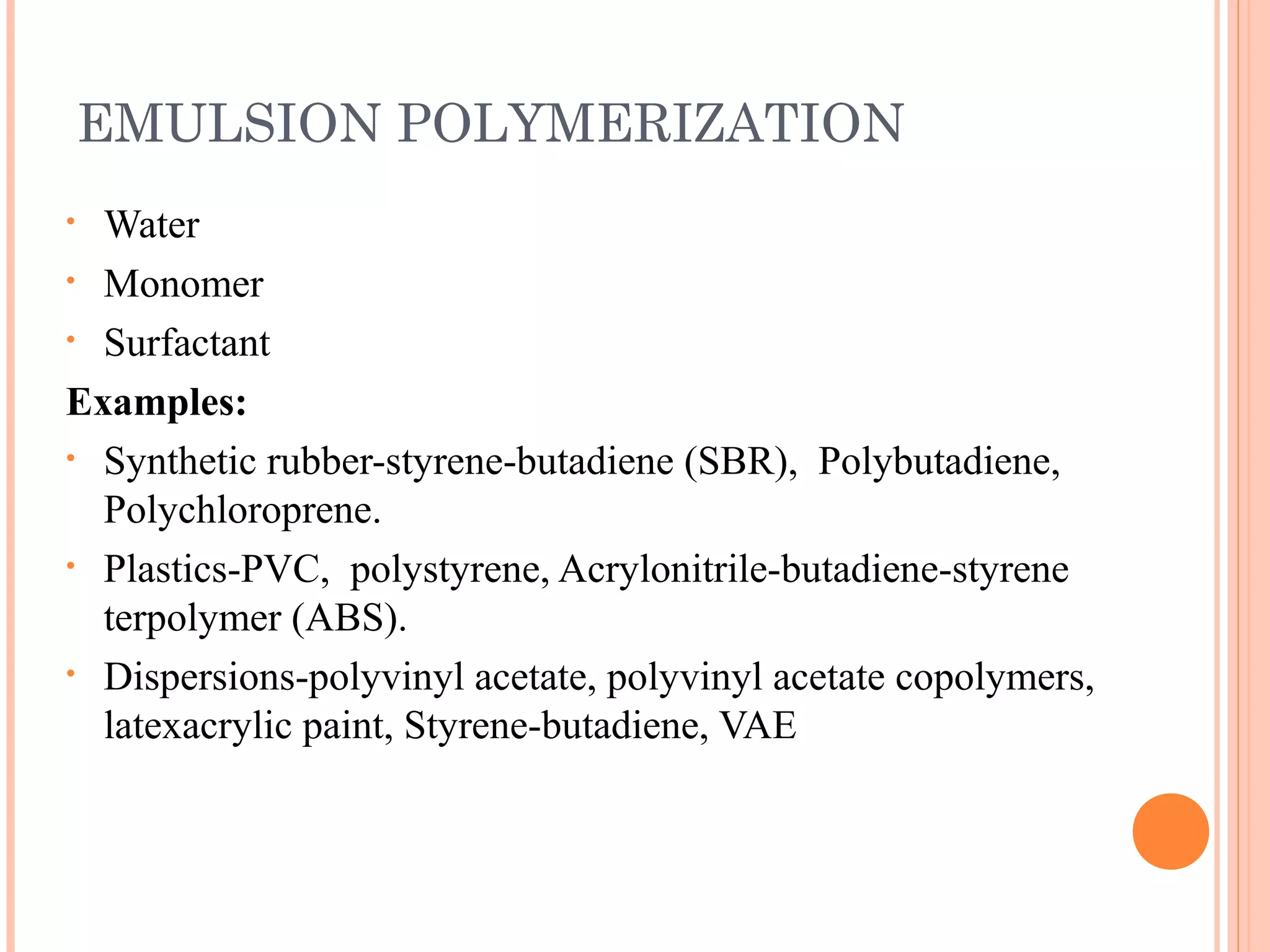
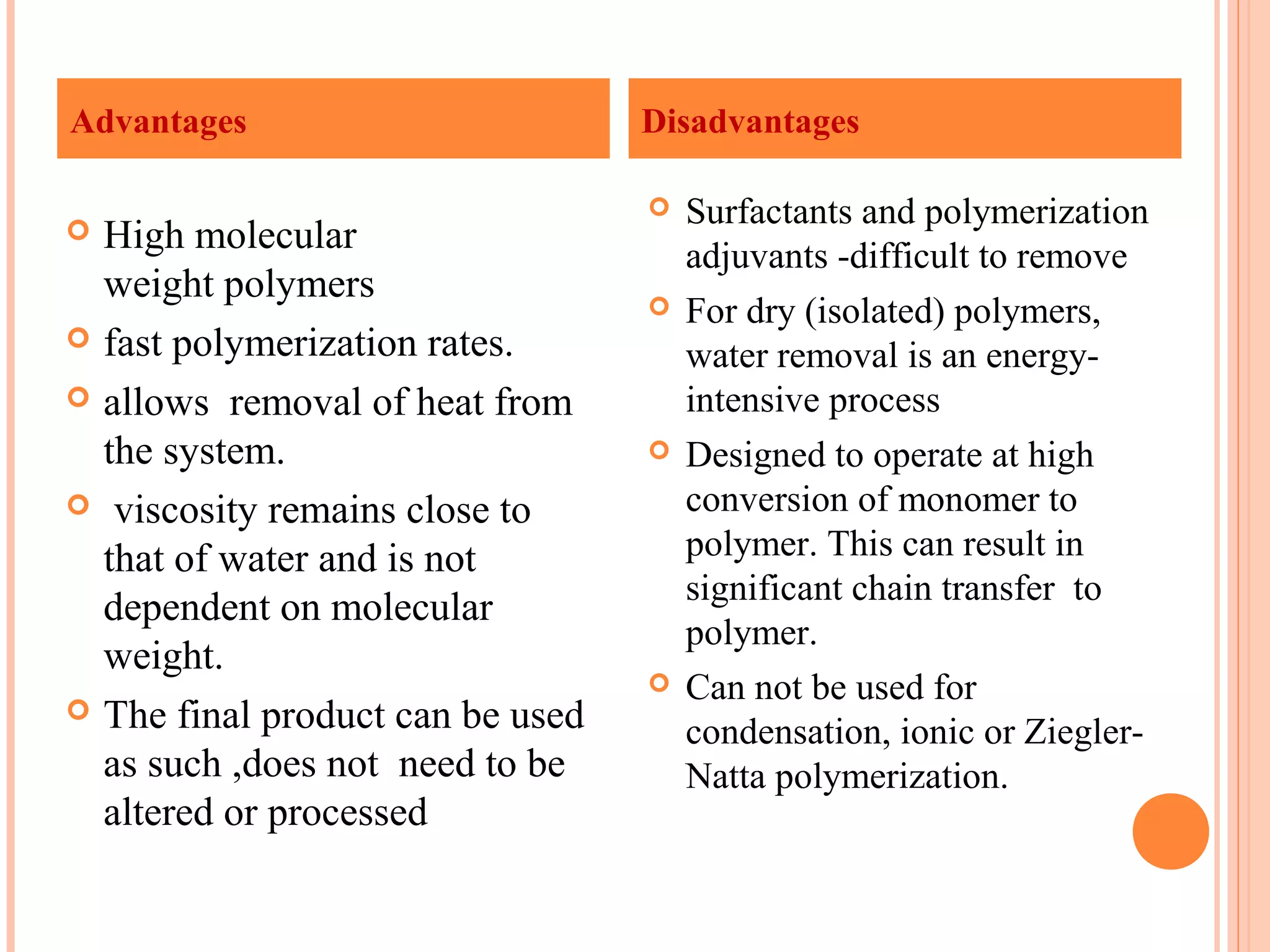
The document discusses various polymerization techniques including addition and condensation polymerization, each with subcategories such as bulk, solution, suspension, and emulsion polymerization. It details the methods, advantages, and disadvantages of each technique, highlighting their applications in producing different polymers like PVC and polystyrene. The document emphasizes the impact of factors like viscosity and temperature control on the efficiency and outcomes of the polymerization processes.