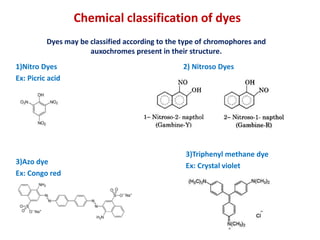
This document provides information about organic chemistry concepts including synthetic dyes, synthetic drugs, and synthetic polymers. It discusses the classification of dyes based on their chemical constitution and provides examples of important synthetic dyes like Congo red, Crystal violet, Phenolphthalein, and Alizarin. It also summarizes theories on the relationship between color and chemical structure, including Witt's theory and the modern electronic concept. Classification of dyes as natural or synthetic is described.