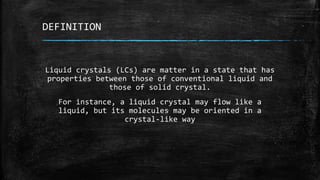
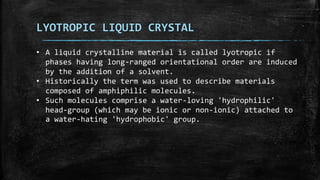
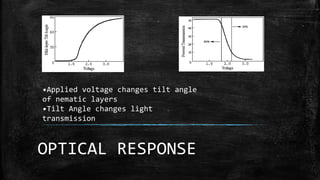
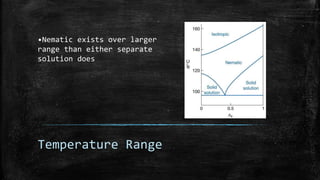
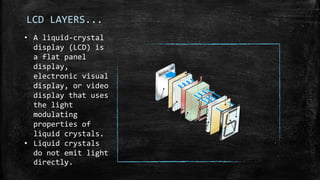
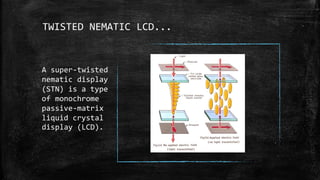
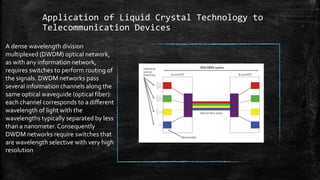
The document discusses liquid crystals, their definition, types, properties and applications. It defines liquid crystals as matter in a state between liquid and solid crystal, possessing properties of both. Liquid crystals are divided into thermotropic, lyotropic and metallotropic types based on the factors inducing their phase transitions. Their properties include orientational order, birefringence, and response to external fields. Common applications mentioned are LCD displays, dyes, advanced materials and drug delivery. Liquid crystal technology finds use in telecommunication switches for routing wavelength division multiplexed optical signals.