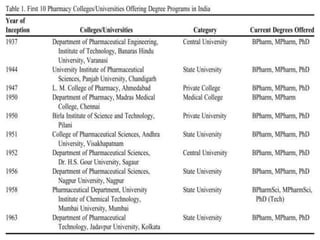
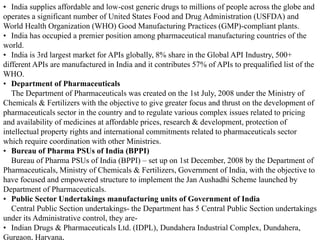
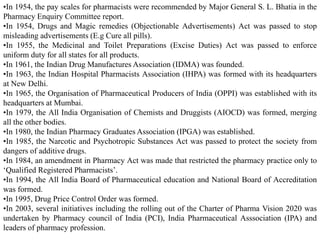
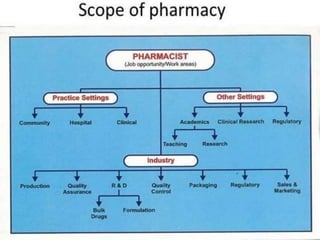
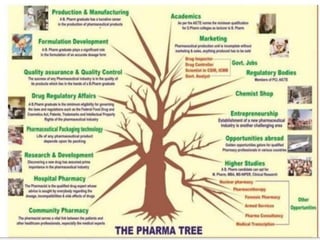
This document provides historical background on the development of the pharmacy profession from ancient times to the present. It discusses how pharmacy originated in ancient cultures using plants and minerals for medicine. Key figures like Hippocrates, Galen, and Avicenna advanced early understandings of medicine and pharmacology. The profession evolved with the establishment of pharmacies, regulations, and the separation of pharmacy and medicine. Pharmacy further developed with advances in chemistry, standardization of medications, and expanded roles for pharmacists in patient care.






























![PHARMACEUITICAL EDUCATION IN INDIA
• A variety of Pharmacy programs/courses are offered in India today:
o Diploma in Pharmacy (D. Pharm.)
o Bachelor of Pharmacy (B. Pharm.)
o Master of Pharmacy (M. Pharm.)
o Master of Science in pharmacy [MS (Pharm)]
o Practice-based Doctor of Pharmacy (Pharm. D.)
o Master of Sciences in Pharmacy [MS (Pharma)]
o Master of Technology in Pharmacy [MTech (Pharm)]
o Doctor of Philosophy in Pharmacy (Ph. D)
o Integration of two courses like B. Pharm+MBA or M. Pharm+MBA has also been
initiated by some institutions.](https://image.slidesharecdn.com/pharmaceutics-1unit-1-221214121936-550b56aa/85/Pharmaceutics-1-Unit-1-pptx-31-320.jpg)












![PRESENT ERA
• Currently, pharmaceutical education in India imparted as D.Pharm ( two year course after
10+2 science), B.Pharm (Four years [8 semester] after 10+2 sciences), B.Pharm (Practice)
2 ears after D,Pharm, Pharm.D , Post graduate course like M.pharm (2 years after
B.Pharm) in various braches such as Pharmaceutics, industrial Pharmacy, Pharmaceutical
Technology, Pharmaceutical Chemistry, Pharmaceutical Analysis, Pharmacology,
Pharmacognosy, Pharmaceutical Quality Assurance, Pharmaceutical Biotechnology,
Phytopharmacy and Phytomedicine, Regulatory Affairs and Pharmacy Practice.
• Student who qualified in GPAT (Graduate Pharmacy Aptitude Test) examination and
admitted to M.Pharm are entitled for scholarship sanctioned by AICTE.
• From inception of the programmes, the curriculam for D.Pharm and Pharm.D has been
same across the country. However, till 2017-18 B.Pharm and M.Pharm curriculum were
varying from university to university. PCI has recently introduced B.Pharm regulation
2014, M.Pharm regulation 2014 , D.Pharm regulation 2020 and accordingly suggested
single syllabus at the national that was implemented by universities.](https://image.slidesharecdn.com/pharmaceutics-1unit-1-221214121936-550b56aa/85/Pharmaceutics-1-Unit-1-pptx-45-320.jpg)