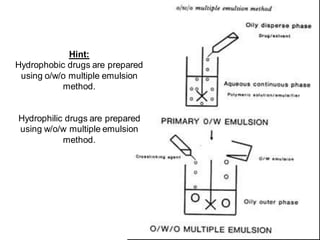
This document provides an overview of pharmaceutical emulsions. It defines emulsions as consisting of two immiscible liquids, one dispersed as small droplets within the other. Emulsions are unstable without emulsifying agents, as the droplets will coalesce over time. The document outlines different emulsion types (O/W vs. W/O), describes formulation methods like multiple emulsions, and discusses factors that influence emulsion stability such as flocculation, creaming, and coalescence. It also provides examples of emulsion applications in drug delivery.